Abstract
Background: Autologous stem cell transplantation (ASCT) has dramatically improved the survival of myeloma patients; however, this approach has significant toxicities and nearly 25% of MM patients progress within one year from their transplant. While gene expression profiling-based (GEP) molecular classification has permitted the identification of unresponsive high-risk patients, these approaches have proven too costly and complex to translate into clinical practice. Less expensive and more readily available methods are needed clinically to identify, at the time of diagnosis, MM patients who may benefit from more aggressive or experimental therapies. While protein-based tissue arrays offer such alternative, biases introduced by the “observer-dependent” scoring methods have limited their wide applicability.
Methods: We have designed a simplified, fully automated and quantitative protein expression based-classification system that will allow us to accurately predict survival post ASCT in a cost effective and “observer-independent” manner. We constructed tissue microarrays using diagnostic bone marrow biopsies of 82 newly diagnosed MM patients uniformly treated with a dexamethasone based induction regimen and frontline ASCT. Using the HistoRx PM-2000 quantitative immunohistochemistry platform, coupled with the AQUA analysis software, we have examined the expression of the following proteins: FGFR3 which is associated with t(4;14), cyclin B2 and Ki-67 which are associated with cellular proliferation, TACI which is associated with maf deregulation, and phospho-Y705 STAT3 and p65NF-κB, which are associated with myeloma cell growth and survival. For FGFR3, patients were divided into FGFR3 positive and negative groups based on hierarchical clustering of their AQUA score. For all other proteins examined, based on AQUA scores, the top quartiles or quintiles of patients were classified as high expression groups. Based on the univariate analysis, patients were further classified as “High Risk” MM if they had been identified as high expressers of either TACI, p65NF-κB or FGFR3. The Kaplan-Meier method was used to estimate time to progression and overall survival. Multivariate analysis was performed using the Cox regression method.
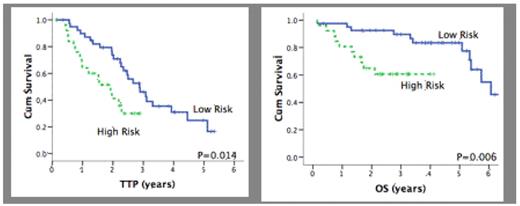
Results: 82 patients were included in this study. In univariate analysis, FGFR3 and p65NF-κB expression were associated with significantly shorter TTP (p=0.018 and p=0.009) but not OS (p=0.365 and p=0.104). TACI expression levels predicted for worse OS (p=0.039) but not TTP (p=0.384). High expression of Ki67 or phospho-Y705 STAT3 did not affect survival. Of the 82 cases, 67 were included in the multivariate analysis since they had AQUA scores available for all markers: 26 (38.8%) were considered as High Risk by their AQUA scores and had significantly shorter TTP (p=0.014) and OS (p=0.006) compared to the Low Risk group. The median TTP for the Low and High Risk groups was 2.9 years and 1.9 years, respectively. The 5-years estimates for OS were 60.6% for the High Risk group versus 83.5% for the Low Risk group. Multivariate analysis was performed using del13q and our risk group classification as variables. Both our risk group classification and del13q were independent predictors for TTP, having 2.4 and 2.3 greater risk of relapse, respectively. Our risk group classification was the only independent predictor of OS with the High Risk group having a 5.9 fold greater risk of death.
Conclusions: We have found that the expression of FGFR3, TACI, and p65NF-κB, in an automated and fully quantitative tissue-based array, is a powerful predictor of survival post-ASCT in MM and eliminates the “observer-dependent” bias of scoring TMAs. A validation of this “High Risk” TMA based signature is currently underway in larger and independent cohorts.
Disclosures: Bahlis:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; OrthoBiotech: Honoraria, Speakers Bureau.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal