Abstract
Background: We previously reported a retrospective study suggesting that erythropoietin therapy starting on day 28 after autologous HCT was highly effective to improve Hb levels and that i.v. iron might improve response in patients with low transferrin saturation (Baron et al., Clin Cancer Res 2003). This prompted us to conduct a multicenter prospective randomized study analyzing the impact of darbepoetin alfa with or without i.v. iron on erythroid recovery after autologous HCT.
Patients and Methods: 127 autologous HCT recipients with lymphoid malignancies were randomized 1:2:2 between no treatment (group 1, n=25), darbepoetin alfa (Aranesp®) 300 μg QOW starting on day 28 after HCT for a total of 7 doses (group 2, n=52), or the same regimen of darbepoetin alfa plus i.v. iron sucrose (Venofer®) 200 mg on days 28, 42 and 56 after HCT (group 3, n=50). Once the target Hb (13 g/dL) was attained, the dose of darbepoetin alfa was reduced to 150 μg, while it was withheld when Hb was ≥ 14 g/dL. Primary endpoints included proportion of complete correctors (i.e. patients reaching Hb □ 13 g/dL) before day 126 post-transplant and median time to achieve Hb correction in each arm. Data were analyzed following the intention-to-treat principle. The proportion of complete correctors by day 126 in each group was compared using the Fisher’s exact test, and median times to reach 13 g/dL in each group were compared using the logrank test.
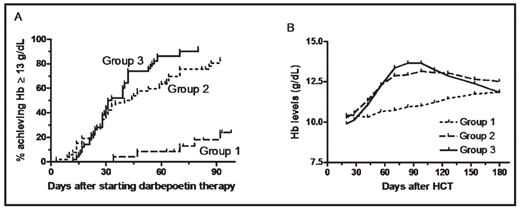
Results: The proportion of complete correctors was 24% in group 1, 81% in group 2 (P<0.001 compared with group 1), and 92% in group 3 (P<0.001 compared to group 1, and P=0.099 compared to group 2). Median time to achieve Hb □ 13 g/dL was not reached in group 1, 42 days in group 2 (P<0.001 compared to group 1), and 32 days in group 3 (P<0.001 compared to group 1 and P=0.127 compared to group 2) (Fig 1A). Hb evolution in each group is shown in Fig 1B. Mean ± standard deviation total doses of darbepoetin-alfa administered were 1,445 ± 489 μg in group 2 vs 1,272 ± 443 μg in group 3 (P=0.06). Ferritin levels at the end of study were 477 ± 597, 393 ± 599 and 479 ± 488 in groups 1, 2 and 3, respectively (NS). Eight patients (2 in group 1, 4 in group 2, and 2 in group 3) required red blood cell transfusions on study, including 4 patients following early disease progression. There was no difference in rates of thrombo-embolic events or other complications among the groups. Quality-of-life data will be presented.
Conclusions: This is the first prospective randomized trial demonstrating that darbepoetin alfa is safe and highly effective to ensure full erythroid reconstitution after autologous HCT when started on day 28 posttransplant. I.v. iron sucrose tended (not statistically significant) to further fasten erythroid recovery with a lower dose of darbepoetin alfa required. Future studies in this setting should aim at further investigating the impact i.v. iron might have on improving response in patients with low transferrin saturation.
Disclosures: Beguin:Amgen Belgium: Research Funding. Off Label Use: Off label use of iron sucrose (VenoferR).
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal