Abstract
The proteasome inhibitor Bortezomib (Bz) is a potent inducer of plasma cell apoptosis via suppression of NFkB activity. Bz is also associated with the dose limiting induction of thrombocytopenia as well as inhibition of dendritic cell (DC) function, which may limit the induction of autologous T cell responses to myeloma antigens in vivo. We hypothesized that the dose, and therefore the adverse effects of Bz, may be reduced by the addition of the agonistic anti-TRAIL-R1 antibody Mapatumumab (Mp). A dose of Bz that did not result in detectable apoptosis was determined for each of the cultured human plasma cell lines (RPMI8226, U266, LP-1, NCI-H929, OPM-2 and JJN3) via titration experiments in which each cell line was cultured for 24–48 hrs in Bz concentrations from 0.1–10nM. Cells were stained with annexin V-FITC and the viability dye 7-AAD and analyzed on a BD LSRII flow cytometer. Plasma cell surface expression of TRAIL-R-1 and -2 were also assessed and correlated with sensitivity to Mp induced apoptosis. Bz monotherapy at 10nM produced 85–98% apoptosis in all plasma cell lines. In contrast 4/6 cell lines were sensitive to Mp alone, RPMI8226 at 0.06ug/ml, U266 and OPM-2 at 1ug/ml and LP-1 at 10ug/ml. When non-apoptosis inducing Bz doses were used in combination with titrated doses of Mp (from 0.01 to 50ug/ml) apoptosis of RPMI8226, U266 and OPM-2 was enhanced by 32%, 30% and 15% respectively compared to Mp alone (p=<0.05 for each cell line). The particular sensitivity of RPMI8226 to Bz/Mp therapy correlated with this cell line’s higher level of TRAIL-R 1 surface expression in comparison to U266. In contrast, the addition of Bz to Mp did not result in any additional apoptosis induction over Mp alone in LP-1, JJN3 and NCI-H929. Conversely, 24 hrs of Bz pretreatment resulted in subsequent enhanced sensitivity of LP-1 to Mp but not NCI-H929. In conclusion, the effective dose of Bz may be significantly reduced when used in combination with Mp. The Bz+Mp combination therapy may be more efficacious than Bz alone and allow dose modification of Bz. Reduction in Bz dose may in-turn reduce side-effects whilst allowing the preservation or promotion of endogenous immune responses.
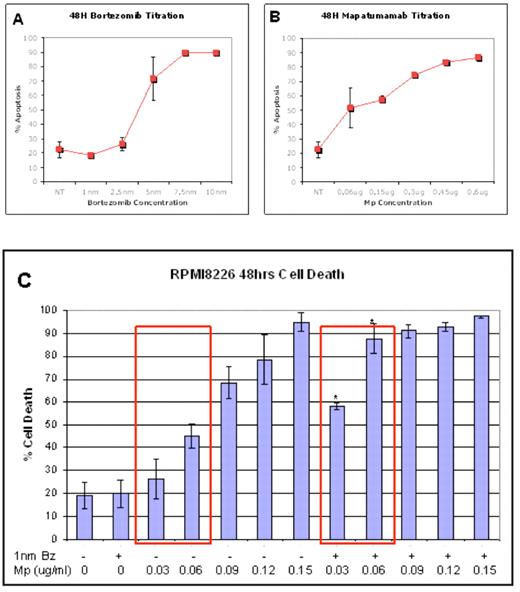
Combination bortezomib and Mapatumamab therapy induce synergistic death of the human myeloma cell line RPMI8226. A dose response study for (A) bortezomib (B) Mapatumamab was performed on RPMI8226 cells by treating the cells with titrated doses of the monotherapy for 48 hours in triplicate wells. Subsequently RPMI8226 cells were treated with combination bortezomib and Mapatumamab therapy (C). Bortezomib dose (1nM) did not induce apoptosis above untreated controls, Mp was added at doses from 0.03 to 0.15ug/ml. A statistical comparison was made between apoptosis with Mp monotherapy versus Bz plus Mp using student t-test, * represents data points where combination therapy is statistically different to Mp alone, p<0.05. Results are representative of two separate experiments.
Combination bortezomib and Mapatumamab therapy induce synergistic death of the human myeloma cell line RPMI8226. A dose response study for (A) bortezomib (B) Mapatumamab was performed on RPMI8226 cells by treating the cells with titrated doses of the monotherapy for 48 hours in triplicate wells. Subsequently RPMI8226 cells were treated with combination bortezomib and Mapatumamab therapy (C). Bortezomib dose (1nM) did not induce apoptosis above untreated controls, Mp was added at doses from 0.03 to 0.15ug/ml. A statistical comparison was made between apoptosis with Mp monotherapy versus Bz plus Mp using student t-test, * represents data points where combination therapy is statistically different to Mp alone, p<0.05. Results are representative of two separate experiments.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal