Abstract
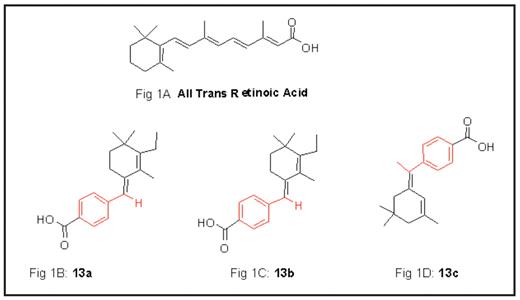
All trans retinoic acid (ATRA) is the treatment of choice for Acute Promyelocytic Leukemia (APL) and binds to the transcriptional repressor PML-RAR-alpha oncogene. The binding of ATRA to RAR-alpha relieves transcriptional repression and leads to induction of genes important for myeloid differentiation. ATRA consists of an acid moiety at position 15 and is also characterized by conjugated double alkene bonds (Fig1A). The exact contributions of these structural components of ATRA in its clinically important binding to RAR-alpha receptor are not very well studied. In an effort to improve the therapeutic index and to study its binding mechanism, we synthesized novel retinoic acid analogs (retinoids). Our objective was to construct retinoids to test these structure functional correlates. 3 novel retinoids created were synthesized as follows:
Compound 13a (Fig1B): consisting of modifying conjugated alkene backbone while keeping acid moiety intact.
Compound 13b (Fig1C): consisting of modifying conjugated alkene backbone and converting acid to ester moiety.
Compound 13c (Fig1D): consisting of modifying conjugated alkene and cycloalkene ring backbone and keeping acid moiety intact.
We tested the ability of these newer retinoids to bind to RAR-alpha as well as inhibit the proliferation of PML-RAR-alpha containing acute promyelocytic leukemic cell line NB4. Reporter assays using a RAR- luciferase demonstrated that Compound 13a successfully relieved transcriptional repression by RAR-alpha and led to downstream gene induction. This was significantly less compared to wild type ATRA demonstrating the importance of C9–C10 double bonds in this binding. Compounds 13b and 13c could not relieve RAR mediated repression of gene induction demonstrating that the acid moiety plays a important role in this binding. Functional studies were consistent with these observations and showed that both ATRA and 13a were able to inhibit the proliferation of APL cells while 13b, 13c were not. Flow cytometry showed that 13a led to partial differentiation of NB4 cells, as evident from CD11b expression after five days of culture. 13b and 13c did not lead to any differentiation, while ATRA led to the greatest expression of CD11b.
Our results demonstrate that both the acid moiety and conjugated double bonds are important in binding to RAR-alpha and the subsequent transcriptional repression by retinoic acid. These insights will be important in future efforts to improve the stability and efficacy of ATRA and also have implication in efforts focused on treating ATRA resistant cases of APL.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal