Abstract
Background: Radio-immunoconjugates such as 90Yttrium labeled ibritumomab tiuxetan (IT) have been shown to have significant activity in the treatment of patients with low grade NHL. Bortezomib (Bz), the first proteasome inhibitor, has also demonstrated anti-tumor efficacy in these patients with the primary toxicities being transient myelosuppression and neuropathy that is mostly reversible. Preclinical data suggest synergy or additive effects with bortezomib and radiation, providing a rationale for combination of IT with Bz.
Methods: Pts with recurrent or refractory, low grade or mantle cell B-cell NHL with good organ function and less than 15% marrow involvement by tumor were eligible for enrollment. Pts received IT according to the standard schedule beginning with 250 mg/m2 rituximab on days 1 and 8 along with indium labeled IT on day 1 and 90Yttrium labeled IT at a dose of .4 mCi/kg on day 8. Bortezomib was given on days 4, 8, 11 and 15. The dose of bortezomib used in cohort 1 was 1.0mg/m2 and the dose was increased to 1.3 and then 1.5 mg/m2 in subsequent cohorts. Three patients were enrolled in cohorts 1 and 2 with 6 pts at dose level 3. The MTD was not reached. IT was given following bortezomib and rituximab. Pts were followed until resolution of toxicities to less than grade 2 and restaged every 3 months. A dose limiting toxicity was considered any grade 4 non-hematological toxicity, any grade 3 non-hematological toxicity lasting >7 days, or any grade 4 hematologic toxicity lasting >4 weeks that was thought to be related to study drug.
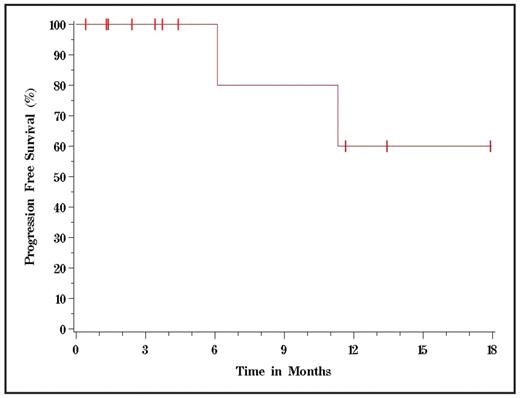
Results: 12 pts, 7 males, 5 females, median age 65, with a median of 3 prior treatments regimens (range 1–6) were enrolled at dose levels 1, 2 and 3. Histologies were reported as follows: follicular (5 pts), mantle cell (5 pts), marginal zone (1 pt), and cutaneous small B cell (1 pt). The most frequently reported toxicities were hematologic with ≥grade 3 thrombocytopenia in 2 patients. Grade 4 febrile neutropenia occurred in one patient and grade 4 neutropenia without fever was seen in two additional pts. Interestingly all grade 4 hematological toxicities occurred in cohort 1 and no grade 3–4 hematologic toxicities have been reported in cohort 3. One DLT occurred in a patient enrolled in cohort 3 who experienced painful grade 3 sensorimotor neuropathy and fatigue. No other DLTs or grade 3 or 4 non-hematologic toxicities were observed in any dose level. So far, 9 of 12 pts have completed all 15 days of therapy and are evaluable for response. CR was achieved in 3 of the 9 pts and PR in 2 for an overall response rate of 56%. Of the 4 remaining evaluable patients 3 achieved SD and 1 had PD. 2 pts at dose level 3 are still being followed for toxicity, and 3 pts have not yet completed restaging post therapy. 2 pts have been taken to transplant, one, who was in a CR prior to transplant, received an allograft. The other patient obtained a PR on the trial and proceeded to an autograft after receiving 2 cycles of ICE (ifosfamide, carboplatin, etoposide) chemotherapy. Although peripheral blood stem cell collection was attempted after pt completed protocol therapy, this pt ultimately had to undergo a bone marrow harvest. Neither pt had difficulty with engraftment after the stem cell transplant. Both pts are in continued CR, 15 and 3 months post transplant, respectively. The median follow-up for the 11 survivors was almost 4 months. One patient with marginal zone lymphoma died due to progression of disease 6 months after completing treatment. One patient with follicular lymphoma is alive, but with disease progression that occurred 11 months after therapy. At 1 yr, OS was 80%, PFS was 60%, and the relapse rate was 25%. 2 pts with mantle cell lymphoma remained progression-free beyond 11 months.
Conclusion: The combination of bortezomib at 1.5 mg/m2 × 4 doses and a single dose of 90Yttrium labeled IT appears to be safe, easy to administer, and effective in a population of heavily pre-treated pts with NHL. A larger Phase II study in less advanced pts appears warranted.
Disclosures: Off Label Use: Bortezomib is FDA approved for relapsed/refractory Mantle cell lymphoma but not for other low grade B cell NHL..
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal