Abstract
Introduction: Appropriate correction of hemostasis in VWD is essential not only to stop acute perioperative bleeding, but also to promote postoperative wound healing. In order to prevent a drop of the VWF plasma level below the hemostatically necessary concentration, repeated infusions of VWF containing concentrates are required. Plasma derived VWF/FVIII concentrates vary, however, in several aspects such as the VWF/FVIII ratio. As therapy of VWD primarily aims at correcting functional VWF level, the question arises, whether VWF/FVIII concentrates differ with regard to the plasma level of FVIII resulting from repeated concentrate infusions.
Methods: The goal of the present study was to compare VWF and FVIII levels after repeated administration of 150 IU (VWF:RCo)/kg (7 doses at 4-hour intervals). Two plasma derived VWF/FVIII concentrates, Humate-P®/Haemate® P and Wilate®, were selected for the comparison. The concentrates were administered as i.v. bolus to CHB rabbits (n=6/group). Blood samples were collected for close monitoring of the resulting substitution levels. The VWF and FVIII levels were quantified using commercially available ELISA assays.
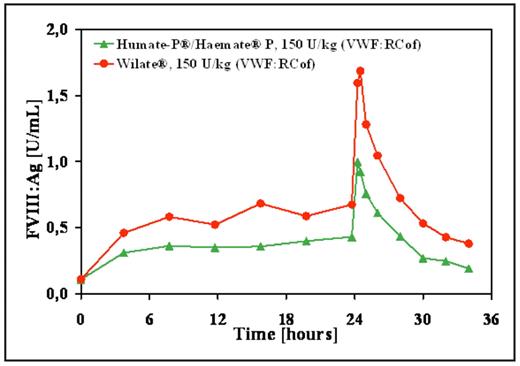
Results: Pharmacokinetic analysis (AUC, Cmax, half-life, MRT, clearance) demonstrated that the time course of VWF plasma levels were similar. The ratio of the VWF AUC of Humate-P®(Haemate® P)/Wilate® was 0.98 and the maximal VWF plasma concentration of Humate-P®/Haemate® P was slightly higher, at a ratio of 1,16. In contrast, the FVIII levels continuously accumulated upon treatment with Wilate® leading to trough levels, which were about 1,6 fold higher compared to Humate-P®/Haemate® P (Figure). Accordingly, also the peak FVIII level was 1,7 fold higher upon treatment with Wilate®, while the half-life of FVIII did not differ substantially between the two concentrates. The FVIII trough level of Wilate® together with its higher peak level resulted in an AUC, which was 2 fold higher (p<0.0001) than observed for Humate-P®/Haemate® P.
Conclusion: Against the background of Wilate® containing a two fold higher FVIII concentration compared to Humate-P®/Haemate® P, such differential effects appear plausible. At nominally equal VWF:RCof repeated doses, the associated AUC with respect to FVIII:Ag of Humate-P®/Haemate® P was significantly lower, as compared to Wilate®. This is obviously due to the lower FVIII content in the Humate-P®/Haemate® P product. Therefore, a lower accumulation of FVIII trough levels was observed for Humate-P®/Haemate® P as compared to Wilate®.
Disclosures: Kronthaler:CSL Behring GmbH: Employment. Stockschlaeder:CSL Behring GmbH: Employment. Dickneite:CSL Behring GmbH: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal