Abstract
High-dose therapy and autologous stem cell transplantation (HDT/ASCT) and rituximab immunotherapy have been increasingly applied in the management of non-Hodgkin’s lymphomas (NHL). Although both approaches have been individually associated with B-cell depletion and hypogammaglobulinemia, the incidence, time course, and predictors of prolonged deficiencies following a combined treatment approach is unknown.
Methods: We completed a series of prospective phase II studies of HDT/ASCT combined with rituximab for patients with relapsed follicular lymphoma (FL) or diffuse large B cell lymphoma (DLBCL). In two phase II trials of patients with FL (R/Tx and R-IFN/Tx), patients received 9 infusions of rituximab 375mg/m2 as both an in vivo purge and as maintenance post HDT/ASCT. In a trial with relapsed DLBCL or transformed lymphoma, patients received 8 infusions with rituximab 375mg/m2 only as part of the salvage chemotherapy regimen (R-ESHAP/Tx). Immunoglobulin levels were expressed as percentages with 100% representing the lower limit of normal at the institutional lab. Hypogammaglobulinemia was defined as <75% of this lower limit. A time-to-recovery from hypogammaglobulinemia was determined by Kaplan-Meier statistics.
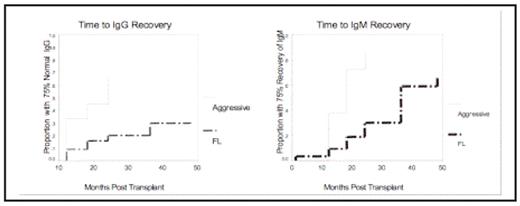
Results: Fifty-one patients with FL were transplanted and were subsequently treated with maintenance rituximab (R/Tx study) or with maintenance rituximab (MR) and interferon-alpha (R-IFN/Tx). Twenty-six patients with relapsed aggressive-histology lymphoma were transplanted with the R-ESHAP salvage regimen. The mean age of the FL patients was 45 and the mean age of the aggressive-histology lymphoma patients was 49. The median number of prior therapies was 1 (range 1–3) for the FL patients and bone marrow involvement at study entry was present in 63%. The median number of prior therapies was 1 (range 1–5) for the aggressive-histology lymphoma patients and bone marrow involvement was present in 15%. Baseline IgG hypogammaglobulinemia was seen in 18% of patients with relapsed FL and 15% of patients with relapsed DLBCL/transformed lymphoma. Median baseline Ig levels in each study are displayed in Figure 1. Patients with FL undergoing HDT/ASCT and MR (combined R/Tx and R-IFN/Tx studies) had prolonged IgG hypogammaglobulinemia compared to patients with aggressive-histology lymphoma (log-rank p=0.0047; see Figure 2). The median time-to-recovery from hypogammaglobulinemia had not been reached after a median follow-up of 42 months. Similarly, IgM level recovery was delayed in the FL group compared to the aggressive-histology patients (p=.0001), although late recovery was noted for this Ig subtype (median time-to-recovery 36 months post-ASCT). Univariate analyses revealed that persistent hypogammaglobulinemia (at 24 months) was associated with FL histology (vs. DLBCL/transformed; p=0.006), bone marrow involvement at study entry (p=0.008), and hypogammaglobulinemia at study entry (p=0.026). Factors not associated with persistent hypogammaglobulinemia included: number of prior therapies, age, and administration of maintenance interferon-alpha. Post-transplant (>3 months) infections included herpes zoster reactivation (n=7) and pneumonia (n=8). One individual in the R-ESHAP/Tx study died of PCP pneumonia 4 months post HDT/ASCT. A relationship between grade III-IV infections and prolonged hypogammaglobulinemia was not evident on univariate analysis (data not shown).
Conclusions: Patients with follicular lymphoma undergoing high dose therapy and stem cell transplantation together with rituximab maintenance are likely to experience a prolonged hypogammaglobulinemia whereas this is less likely with patients with aggressive-histology lymphoma undergoing similar doses of rituximab as part of their salvage therapy. Further research is required to elucidate the relative contributions of disease histology, bone marrow involvement, baseline hypogammaglobulinemia, timing of rituximab infusions or other factors not yet identified.
Disclosures: Off Label Use: rituximab for in-vivo purge and post-transplant maintenance.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal