Abstract
Dasatinib, as a 2nd generation tyrosine kinase inhibitor, is an effective Bcr-Abl kinase inhibitor for chronic myeloid leukemia (CML) with more than 300-fold and 20-fold potency than imatinib and nilotinib, respectively. Through a series of phase I and II clinical trials, dasatinib has demonstrated durable efficacy in CML patients with resistance, suboptimal response, or intolerance to imatinib. However, some adverse effects from dasatinib therapy have been reported in several studies. Among them, pleural effusion is one of the most common adverse effects, and was reported in 15 to 30% of patients.
We investigated the development of pleural effusion from 64 Korean patients registered in a single center for a phase II study which was approved by the institutional review committee and carried out in accordance with the Declaration of Helsinki with informed consent provided from patients. All the patients are under dasatinib therapy after failure of imatinib treatment (22 patients with intolerance and 44 patients with resistance). Median age was 44 years old (range, 18 to 65) and median duration of dasatinib therapy was 29 months (range, 0.5 to 41). The patients were in different phases including 30 in chronic phase (CP) and 30 in accelerated phase (AP) when they started dasatinib treatment.
We found that 23 patients (36%) experienced pleural effusion of any grade (grade 1/2 in 22, grade 3/4 in 1) at least one time. Among the 23 patients, 14 (61%) patients experienced recurrent pleural effusion, and two of them showed a change in grade from grade 1 to 2. The median time to the first occurrence of pleural effusion was 18 weeks (range, 1 to 132), developing within the first 6 months and 12 months of treatment in 13 patients (57%) and in 15 patient (65%), respectively, while the adverse effect occurred even after 28 months (2.3 years) of treatment in 3 patients (13%).
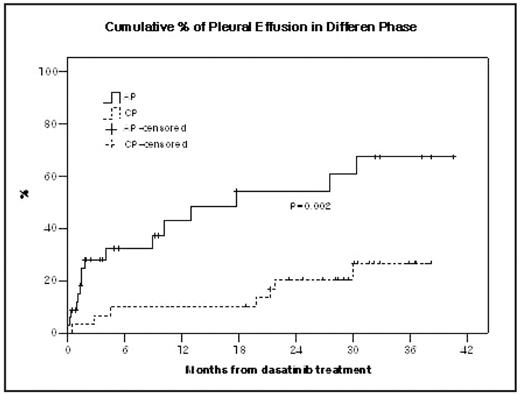
Age, gender, previous interferon a treatment, duration of imatinib treatment, and duration of dasatinib treatment were not significantly different between patients without pleural effusion and patients developed pleural effusion. However, disease phase when dasatinib therapy started showed significant difference (P=0.002) in the development of pleural effusion. Patients in AP showed higher cumulative % of pleural effusion in comparison with those in CP (16/34, 47% vs. 7/30, 23%).
Daily dose amount and dose schedule also gave an influence on occurrence of pleural effusion. We used 4 different categories in dose amount and dose schedule; 100 mg QD (n=3), 140 mg QD (n=32), 50 mg BID (n=7) and 70 mg BID (n=22). Frequency of pleural effusion was higher in the patients treated with BID schedule than in QD schedule (56% vs. 22%, P=0.005), and also higher in the patients treated with 140 mg per day than in 100 mg per day, but not significant (39% vs. 20%, P=0.23). Among 4 categories, 70 mg BID showed relatively higher % of pleural effusion (64%, 14/22, P=0.006).
To see whether dose amount or dose schedule can influence on efficacy, we investigate cytogenetic responses from patients. Portion of complete cytogenetic response (CCyR) was not significantly different in 100 mg doge group and 140 mg dose group (67% vs. 64%). Time to achieving CCyR showed no significant difference (P=0.21) between 100 mg dose group (median 3 months, range; 2 to 17) and 140 mg dose group (median 6 month, range; 1 to 18). In addition, dose schedule did not make any significant difference in portion of achieving CCyR between QD group and BID group (60% vs. 59%).
Based on the observed characteristics of pleural effusion and analysis of cytogenetic response in this study, lower dose (100 mg) administration by QD can be proposed for dasatinib therapy to reduce any possible occurrence of pleural effusion.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal