Abstract
Background: Immunosuppressive therapy (IST) is the therapeutic alternative for patients ineligible for allogeneic transplant. Although the combination of anti-thymocyte globulin (ATG) and cyclosporine-A (CsA) is considered the “gold standard” for these patients, the essentiality of using both drugs can be challenged.
Objectives: This study aims to compare between treatment with ATG + CsA and ATG alone for patients with severe aplastic anemia (SAA), and with non-severe aplastic anemia (NSAA).
Methods: Systematic review and meta-analysis of randomized controlled trials of patients with aplastic anemia comparing ATG and CsA with ATG alone. The Cochrane Library, MEDLINE, conference proceedings and references were searched until 2008. Outcomes assessed were: all-cause mortality, overall hematological failure, refractory disease. Relative risks (RR) with 95% confidence intervals (CIs) were estimated and pooled.
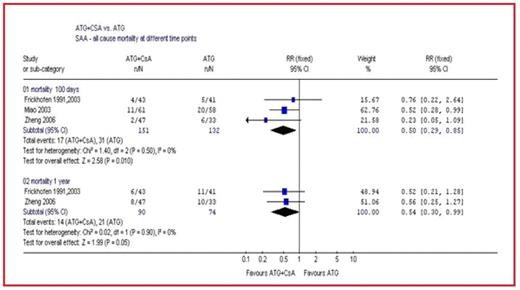
Results: Our search yielded 4 trials. For patients with SAA there was a significant reduction in all-cause mortality at 3 months in the ATG+CsA arm compared with the ATG alone arm (RR 0.50 [95%CI 0.29–0.85]). This effect was also shown at 1 year (RR 0.54 [95%CI 0.30–0.99]) (Figure) and at 5 years (RR 0.58 [95%CI 0.36–0.93]). There was also a reduction in overall hematological failure (RR 0.67 [95% CI 0.49–0.90]) and in the number of patients with refractory disease (RR 0.51 [95%CI 0.33–0.81]) in the ATG+CsA arm.
In patients with NSAA, there was no difference in mortality at 6 months (RR 1.03 [95%CI 0.07–15.78]) and at 5 years (RR 1.03 [95%CI 0.07–15.78]) or in refractory disease (RR 0.89 [95% CI 0.40–1.99]), when ATG+CsA was compared to ATG alone. However, there was a reduction in overall hematological failure in the ATG+CsA arm (RR 0.70 [95% CI 0.42–0.88]).
Conclusions: Our review demonstrates that the combination of ATG with CsA significantly reduces short and long term mortality in patients with severe but not with non severe aplastic anemia. The combination of both drugs should therefore be considered the gold standard only for patients with SAA.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal