Abstract
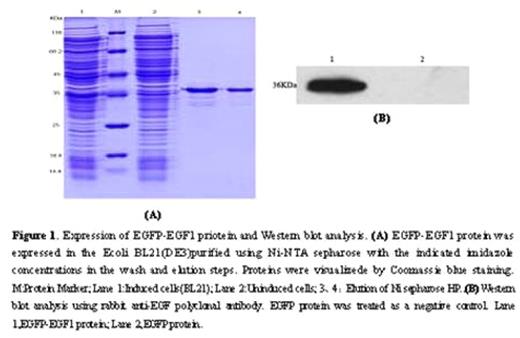
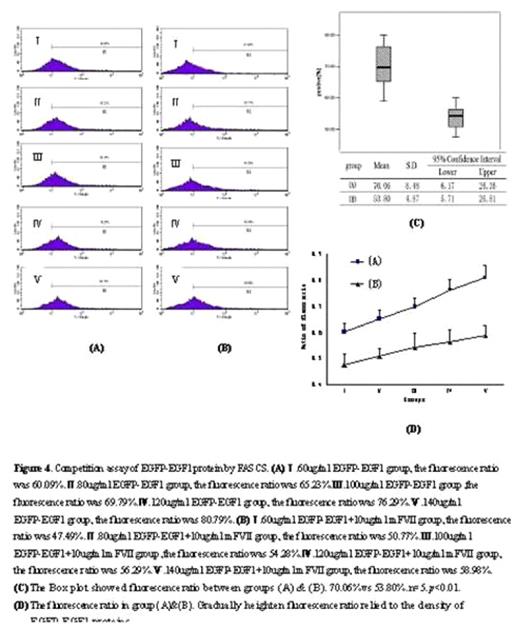
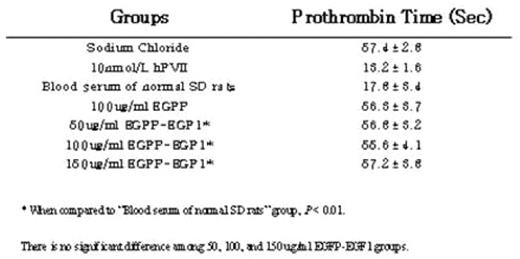
Abstract: The first epidermal growth factor-like (EGF1) domain of coagulation factorVII (FVII) is essential for binding with tissue factor (TF) in human. We hypothesized that the function of rat FVII (rFVII) EGF1 domain might be the same as the human being’s. The clone and expression of rFVII EGF1 domain would be helpful in studying the anticoagulant drugs based on FVII/TF interaction. Accordingly, the model of rat TF expression in vitro was established by lipopolysaccharide(LPS) induction. We amplified the EGF1 domain from a rat liver by reverse transcriptase PCR (RT-PCR), and then a fusion expression vector named pET28a-EGFP-EGF1 was constructed. After expression in Ecoli BL21 cells, the EGFP-EGF1 fusion proteins were analyzed for binding affinity to rat TF by confocal microscope and flow cytometry. Prothrombin time assay was employed to test biological activity of the fusion protein by using FVII depleted human plasma. The resulting plasmid expressed fusion protein EGFP-EGF1 in the soluble form in E.coli BL21, and the recombinant protein was purified successfully with a major band at 36KD in SDS-PAGE(Figure 1). The purified EGFP-EGF1 fusion proteins could definitely depress recombinant mouse FVII((rmFVII) binding with rat TF(rTF)(70.06%VS 53.80%, P< 0.01)(Figure 2 &3). However, the proteins lost the activity of coagulation when compared to that of blood serum of normal SD rats (56.8sVS 17.8s, P< 0.01). (Table 1)We conclude that the rat EGF1 region could specifically bind to TF without the ability of coagulation, and it might facilitate the development of molecular target study in anti-thrombosis treatment.
Table 1. Prothrombin Time test of Fusion proteins
Disclosures: Hu: National Basic Research Program of China (973 Program, No.2007CB935803): Research Funding.
Author notes
Corresponding author



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal