Abstract
Introduction: Some patients treated with EPO and oral iron supplementation respond poorly to EPO, possibly due to inflammation-induced iron sequestration. In response to inflammation, hepcidin, a hormone secreted by the liver, binds to and downregulates ferroportin, the principal cellular iron exporter. In turn, ferroportin downregulation leads to sequestration of iron in absorption sites within duodenal enterocytes, hepatocytes, and in macrophages that break down senescent red blood cells and normally recycle iron back into circulation. This sequestration results in reduced amounts of plasma iron that is bio-available for erythropoiesis. Patients with rheumatoid arthritis (RA), malignancies, or infections frequently manifest inflammatory-associated anemia and elevated hepcidin levels.
Objective: To assess the impact of EPO treatment on hepcidin levels in patients with RA.
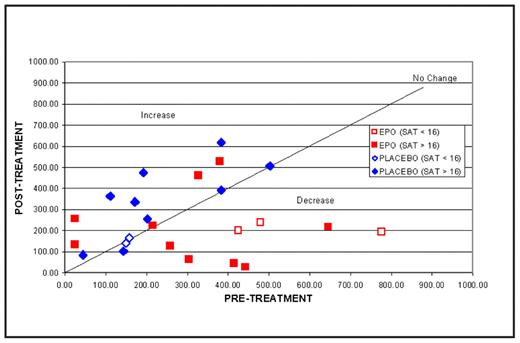
Methods: Serum hepcidin levels and iron status were analyzed from a randomized, double-blind, placebo-controlled, investigational study of 29 patients with RA treated with either EPO or Placebo for 20 weeks. Serum hepcidin levels were measured at baseline and at the end of study. Serum transferrin saturation, serum ferritin, serum transferrin receptor (sTfr) levels and hemoglobin (Hb) concentrations were also measured. Patients with baseline Hb ≤11 g/dL were treated with EPO starting doses of 20,000 U subcutaneously (SQ) once weekly (QW) that could be titrated up to 40,000 U SQ QW. Oral iron was administered to keep the sTfr index <1.5. Iron restriction adequate to hinder erythropoiesis was defined by a transferrin saturation of <16%. Serum hepcidin levels of ≥300 ng/mL were considered elevated. Hematologic response to EPO was defined by achieving a Hb of ≥12 g/dL or a ≥2 g/dL increase in Hb from baseline to the end of study.
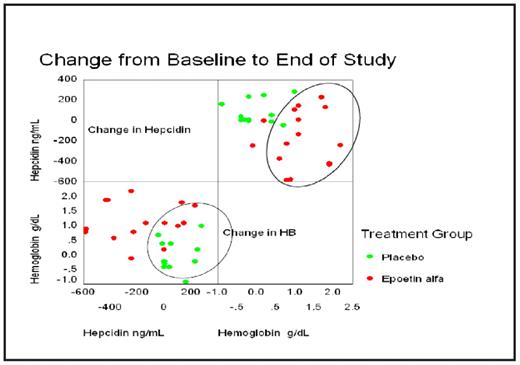
Results: Iron restriction was noted in 5/15 (33%) EPO-treated patients and in 2/14 (14%) receiving Placebo. Hepcidin levels were elevated in 10/15 (67%) EPO-treated patients and in 5/13 (38%) receiving Placebo. There was a strong correlation between baseline serum hepcidin and serum ferritin levels (R= 0.5598 P=0.0019), but not serum transferrin saturation. Eleven of 15 (73%) EPO-treated patients had a Hb response compared to one of 14 (7%) receiving Placebo. Mean hepcidin levels decreased significantly from baseline in the EPO-treated patients (−155.6±186.6 EPO, 90.28±83.1 Placebo, P<0.01). As shown in the diagrams below, serum hepcidin levels appeared to decrease in EPO-treated patients, but not in those receiving Placebo, while Hb levels increased in EPO-treated patients but not in the patients receiving Placebo.
Conclusion: EPO treatment may effectively elicit Hb response in patients with RA and may be associated with suppression of inflammation-induced serum hepcidin levels. Further study is warranted in conditions associated with inflammation, such as cancer and chronic renal failure, in which EPO is used to treat anemia.
Disclosures: Moyo:Centocor Ortho Biotech Services, LLC: Employment. Kersting:Centocor Ortho Biotech Services, LLC: Employment. Westerman:Centocor Ortho Biotech Services, LLC: Consultancy, Research Funding. Langholff:Centocor Ortho Biotech Services, LLC: Employment. Rastogi:Centocor Ortho Biotech Services, LLC: Employment. Mundle:Centocor Ortho Biotech Services, LLC: Employment. Off Label Use: Epoetin alfa in rheumatoid arthritis.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal