Abstract
Introduction: Early response rates to non-myeloablative therapy are encouraging, however long term remissions remain elusive. Manipulating donor lymphocyte infusions (DLI) to preferentially infuse Natural Killer (NK) cells, typically comprising 3–5% of a DLI graft, may promote better antitumor and anti-infective surveillance while reducing risk of graft versus host disease (GVHD). We investigated the feasibility of providing NK cell-enhanced DLI following T cell-depleted non-myeloablative allogeneic transplants. Methods: Patients underwent an alemtuzumab and fludarabine-based non-myeloablative preparative regimens from a 3-6/6 HLA matched related donors. At 6 weeks posttransplant, those who engrafted and did not have sevee GVHD received NK cell-enhanced DLIs, repeated x2 at 8-week intervals. For DLI, NK cells were enriched in a single step using the CliniMACS CD56 Reagent and CliniMACSplus instrument, per manufacturer’s protocols (Miltenyi Biotec Inc, Auburn, CA). The total cell dose infused in patients receiving HLA-mismatched DLI was no more than 0.5 X 106 CD3+CD56neg cells/kg patient weight. Patients receiving HLA-matched DLI (6/6) received no more than 106 CD3+CD56neg cells/kg patient weight.
Analysis: The primary endpoints for feasibility were mortality, occurrence of severe acute GVHD or other unacceptable toxicity, response and duration of response. Efficacy was measured by Progression Free Survival (PFS) and Overall Survival (OS). NK cell function was used as a primary endpoint for immune recovery. NK cell function was measured by flow cytometry using methods that we had previously validated using unfractionated PBMC and CD56+-enriched NK cell preparations.
Results: The NK cell selections worked well with only one device failure resulting in low purity. NK cell purity was 92%+/− 3.5 and yield 74% +/− 16. The resulting cell preparations had low frequencies of CD4+, CD8+ and gamma-delta T cells.
Table 1- Clinical feasibility of enhancing DLIs for NK cells using the Miltenyi system.
| . | . | % PURITY . | % YIELD . | CD3+CD56-/KG × 10e5 . | TOTAL CD56+10e7 . | CD3+CD56 KG × 10e6+/ . | CD3-CD56+ ×10e6 . |
|---|---|---|---|---|---|---|---|
| Median | 95.32 | 83.44 | 5.34 | 1.12 | 1.94 | 9.21 | |
| St Dev | 7.96 | 21.35 | 10.46 | 0.65 | 2.22 | 7.91 | |
| Mis | Median | 97.46 | 77.80 | 2.74 | 1.44 | 3.67 | 9.21 |
| St Dev | 3.24 | 24.05 | 7.79 | 0.61 | 2.41 | 5.56 |
| . | . | % PURITY . | % YIELD . | CD3+CD56-/KG × 10e5 . | TOTAL CD56+10e7 . | CD3+CD56 KG × 10e6+/ . | CD3-CD56+ ×10e6 . |
|---|---|---|---|---|---|---|---|
| Median | 95.32 | 83.44 | 5.34 | 1.12 | 1.94 | 9.21 | |
| St Dev | 7.96 | 21.35 | 10.46 | 0.65 | 2.22 | 7.91 | |
| Mis | Median | 97.46 | 77.80 | 2.74 | 1.44 | 3.67 | 9.21 |
| St Dev | 3.24 | 24.05 | 7.79 | 0.61 | 2.41 | 5.56 |
Ten patients enrolled had HLA-matched (6/6) sibling donors. Of these, 3 had AML, 2 ALL, 3 follicular lymphoma/CLL, 1 myeloma, and 1 myeloproliferative disorder. At entry, six had active disease, 3 were in 2nd CR and 1 was in 1st CR with high risk ALL. These patients received a total of 15 NK cell-enhanced DLI. Infusions were well tolerated with 2 cases of overall grade 2 (grade 3 skin; grade 1 gut), and one case of grade 3 GVHD (grade 3 skin; grade 1 gut and liver). Four of 10 remain alive and in continuous complete remission. Fourteen patients enrolled had HLA-mismatched (3-4/6) related donors. Six had AML, 3 transformed AML, 2 ALL, 1 T cell NHL, 1 myeloproliferative disease and 1 severe aplastic anemia. At time of transplantation, only 1 subject was in CR1, 7 were in CR2, 6 were relapsed/refractory. These patients received a total of 27 NK cell enhanced- DLI. Despite the HLA mismatch, the infusions were well tolerated with 4 cases of overall grade 1 GVHD (primarily skin), 2 grade 2, and 1 grade 4 (gut and liver). Infections were a concern with 1 patient dying of infection while 2 others experienced sepsis. Further, 3 had parainfluenza, 1 VZV, and 2 polyoma virus in the bladder. Eight patients remain alive and 7 are in continuous remission. NK cell function was measured in 22 patients (Figure 1).
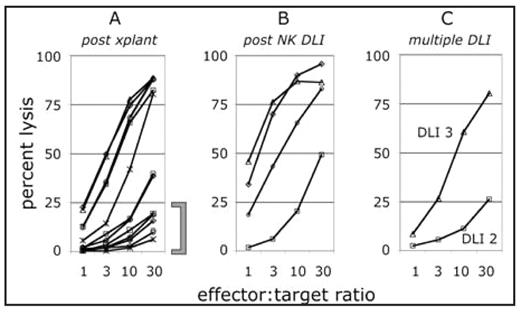
(A) At 6 to 8 weeks post-transplant, some NK cell function had returned in 7 of 22 patients. Among other patients, 7 patients demonstrated low NK cell function (bracket) while 8 others did not recover lymphocytes (not shown). (B) The impact of NK DLI was monitored in 7 patients that had not previously responded. Of those patients, 4 responded within 6 to 8 weeks post-DLI. (C) In one patient, NK cell function returned gradually following a 2nd and 3rd DLI.
(A) At 6 to 8 weeks post-transplant, some NK cell function had returned in 7 of 22 patients. Among other patients, 7 patients demonstrated low NK cell function (bracket) while 8 others did not recover lymphocytes (not shown). (B) The impact of NK DLI was monitored in 7 patients that had not previously responded. Of those patients, 4 responded within 6 to 8 weeks post-DLI. (C) In one patient, NK cell function returned gradually following a 2nd and 3rd DLI.
Conclusion: NK cell enhanced DLI can be safely delivered following T cell depleted nonmyeloablative allogeneic transplantation. Subsequent infusions may allow for improved function. Longer follow up is needed to evaluate affects on long term toxicity and durability of response.
Disclosures: Off Label Use: The Miltenyi system and CD56 selecting antibody are used on an approved IND and are not approved devices or agents yet.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal