Abstract
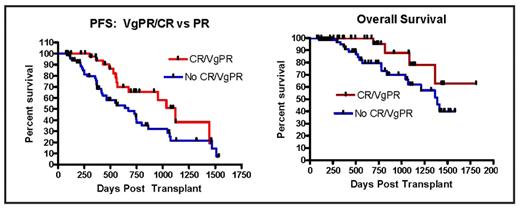
Previous randomized trials evaluating the benefit of high-dose Melphalan and ASCT for MM patients receiving conventional induction chemotherapy have demonstrated that patients who achieve either a CR or VgPR post transplant have a superior PFS and overall survival (OS) compared to those who do not. We report on our 6-year experience comparing the outcome benefit of newly diagnosed MM patients who received either conventional VAD versus IMiDs based induction pre-transplant therapy and the impact on response on 112 consecutive patients transplanted between 5/2002 through 2/2008. The median age was 58 years (33–74). Disease status was Stage III in 92 (82%), Stage II in 11 (10%) and Stage in 9 (8%) patients. Conventional VAD induction chemotherapy was administered pre-transplant in 36 patients and IMiDs (thalidomide, lenalidomide) based therapy in 76 patients. Single ASCT was administered in 78 patients and Tandem ASCT in 34 patients (8 received conventional therapy and 26 received IMiDs, p=NS). Pre-transplant achievement of CR/VgPR was observed in 1/36 (1%) of patients receiving VAD chemotherapy and in 15/76 (20%) of patients receiving IMiDs (p=.017). Post-transplant, 5/36 (14%) patients that received VAD conventional therapy and 39/76 (51%) that received IMiDs achieved CR/VgPR (p=.002). Patients that entered into a CR/VgPR had both a superior PFS and OS (Median PFS 1,118 days vs. 670 days, p=.02/HR.49; Median OS “Not achieved” vs. 1,387 days, p=.05/HR 0.36). Single vs. Tandem transplant had no impact on outcome. These observations support the use of IMiDs as pre-transplant induction therapy over historical VAD chemotherapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal