Abstract
Background: There is a role of novel preparative regimens to further improve the outcome after high-dose chemotherapy and autologous hematopoietic stem cell transplantation (ASCT) in multiple myeloma. Arsenic trioxide (ATO) has synergistic activity with melphalan and ascorbic acid (AA) both in vitro and in vivo, and this 3-drug combination was found to be safe and feasible in both conventional and high-dose settings. Bortezomib, a proteasome inhibitor, is an active agent in newly diagnosed or relapsed multiple myeloma in conventional therapy setting. We conducted a randomized phase II trial to determine the safety and efficacy of a combination of ATO, AA and melphalan with or without bortezomib as preparative regimen in patients with myeloma.
Methods: Sixty patients were enrolled and 58 patient received ASCT between October 2006 and September 2007. One patient developed respiratory failure and another developed extensive pulmonary embolism after enrollment and did not proceed to ASCT. All 58 evaluable patients received melphalan 100 mg/m2 IV on days -4 and -3, AA 1000 mg/day IV on days -9 to -3 and ATO 0.25 mg/kg IV on days -9 to -3. Patients were randomized to 3 arms; no bortezomib (arm 1), bortezomib 1 mg/m2 on days -9, -6 and -3 (arm 2), and bortezomib 1.5 mg/m2 on days -9, -6 and -3 (arm 3). Patients were also asked to fill out a standardized Quality of Life (QOL) questionnaire at enrollment and at 6 months post-ASCT.
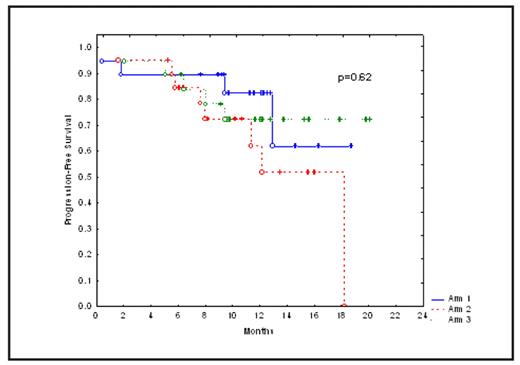
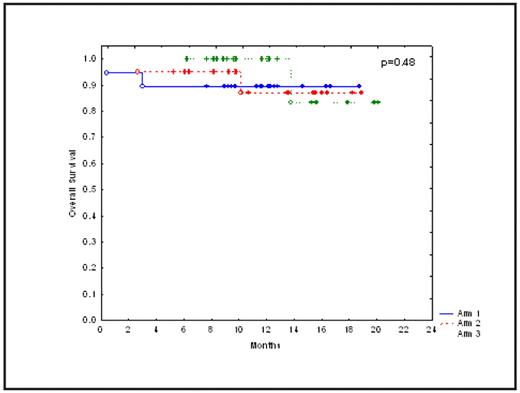
Results: Median age of patients in arms 1, 2 and 3 were 61, 59 and 64 years, respectively (p=0.08). Median interval between diagnosis and ASCT were 12.2, 9.6 and 8.8 months, respectively (p=0.3). Cytogenetic abnormalities were detected in 2, 5 and 8 patients in arms 1, 2 and 3, respectively (p=0.08). With a median follow up of 11.4 months (range 5 to 20) post ASCT in surviving patients, cumulative non-relapse mortality was1.7%. Grade 3–4 toxicity was seen in 6 patients in arm 1 (mucositis 3, dyspnea 1, acute renal failure 1, pleural effusion 1), 6 patients in arm 2 (mucositis 3, diarrhea 1, pneumonia 1 and hydronephrosis 1) and 6 patients in arm 3 (pulmonary edema 2, mucositis 1, intestinal obstruction 1, low back pain 1, elevated transaminases 1) (p=0.9). The most common adverse events were nausea, diarrhea and pedal edema. Grade 1–2 weight gain due to fluid retention was seen in 84, 70 and 95% of patients in arms 1, 2 and 3, respectively (p= 0.1). Median time to neutrophil engraftment (ANC >500/dl) was 10 days in each arm. Complete response rates in arms 1, 2 and 3 were 26, 10 and 16%, respectively (0=0.4). Kaplan-Meiers estimates of progression-free survival (PFS) and overall survival (OS) are shown in Figures 1 and 2. Median PFS and OS have not been reached. There was no significant difference in CR, PFS or OS between the 3 arms (p = 0.4, 0.62 and 0.4, respectively). Relapsed disease at ASCT (p=0.003), >12 months interval between diagnosis and ASCT (p=0.05), and cytogenetic abnormalities at diagnosis (p=0.005) predicted a shorter PFS. On a subset analysis, there was no significant difference in PFS or OS between the 3 arms in patients undergoing ASCT in first remission. Fifty-patients submitted their responses to the QOL questionnaire. More patients at 6-month post ASCT reported better overall health (56% vs.12%, p=0.0001). In contrast, more patients at study enrollment reported moderate to severe pain (44% vs. 27%, p=0.09) and debilitating emotional problems (42% vs. 23%, p=0.07).
Conclusions: Adding bortezomib to ATO, AA and high dose melphalan is safe and well tolerated as preparative regimen for ASCT in patients with multiple myeloma. Patients with chromosomal abnormalities were predominantly treated in the bortezomib arms without an adverse impact on PFS or OS. Overall QOL indicators showed improvement at 6-month post ASCT. Longer follow up is needed to assess the efficacy of this combination.
Disclosures: Qazilbash:Cephalon: Honoraria, Speakers Bureau. Weber:Millenium: Honoraria, Research Funding. Orlowski:Millennium Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal