Abstract
Second generation tyrosine kinase inhibitors (2G-TKI) have displaced allogeneic stem cell transplant as the preferred therapy for patients with CML in chronic phase (CP) who fail imatinib. However a significant proportion of patients still fail to respond to 2G-TKI and may benefit from alternative therapy (including stem cell transplant). We have performed univariate and multivariate analyses in our cohort of 80 patients treated with dasatinib (n=67) or nilotinib (n=13) while still in first CP after imatinib failure in order to identify those patients who will benefit most with these therapies.
The median age was 50 years and 46% were male; 72 patients were resistant to imatinib (2 primary haematological resistance, 40 primary cytogenetic, 32 secondary cytogenetic and 25 developed secondary hematologic resistance) and 8 were intolerant. 20 had developed kinase domain mutations while on imatinib therapy. 31 and 29 patients received maximal doses of imatinib 600 and 800 mg per day respectively. The median follow up was 28.3 months (range 6–42).
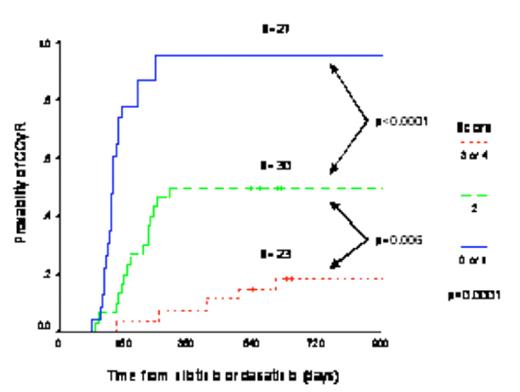
The 3-year cumulative incidence of CCyR was 52.6%. The multivariate analysis identified four pre-2G-TKI independent predictive factors for CCyR, namely low Sokal risk score at diagnosis, the best cytogenetic response obtained on imatinib, G-CSF requirement during imatinib therapy and time from detection of imatinib failure (as defined by European LeukemiaNet criteria) to onset of second 2G-TKI therapy. Using these factors we devised a scoring system that could be used to predict the probability of achieving CCyR on 2G-TKI therapy. The score was calculated by allocating one point when any one of the following four features was present: (1) intermediate or high Sokal risk group, (2) need of G-CSF support during imatinib therapy, (3) institution of 2G-TKI more than 18 months after imatinib failure, and (4) failure to achieve a cytogenetic response on imatinib (≥95% Ph-pos). The 3-year cumulative incidence of CCyR for patients with 0–1 points was 95.6%, with 2 points 50% and with 3–4 points 18.7% (p<0.0001, Figure 1).
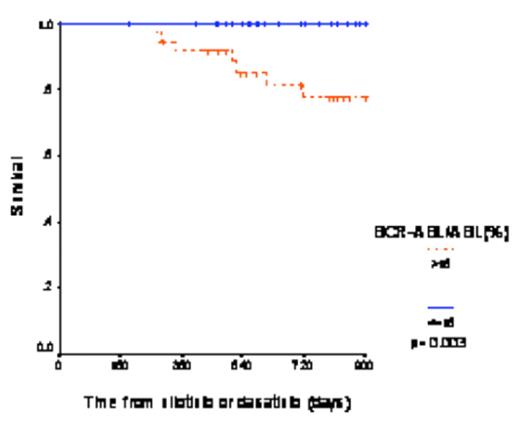
For the 80 patients the probability of 3-year survival was 89.6%. We performed a 3-month landmark analysis to study the relationship between molecular response and subsequent outcome. The 44 patients with a BCR-ABL1/ABL ratio less than 15% at 3 months had a 3-year overall survival of 100% while the 36 patients with a ratio >15% had a survival of 77.4% (p=0.003, Figure 2). We performed a multivariate analysis including all relevant variables defined at the start of 2G-TKI and the 3-month transcript level. The 3-month transcript level was the only independent predictor for survival.
Similarly we performed a 6-month landmark analysis where we explored the relationship between cytogenetic response and outcome. Patients who had achieved a McyR (n=38) or a CCyR (n=32) had a significantly better survival than those with lower levels of cytogenetic response (100% vs. 79.2% (p=0.006) and 100% vs 82.6 (p=0.02)) respectively. We also performed a multivariate analysis including the variables defined at the initiation of therapy, the 3-month transcript levels and the cytogenetic response at 6 months. Interestingly the 3-month molecular response was the only independent variable predicting for survival. Similar results were found for progression-free survival (data not shown).
We conclude that factors measureable before starting treatment with 2G-TKI may be valuable for predicting response; molecular responses at 3-months and cytogenetic responses at 6 months provide further information about the value of continuing treatment with 2G-TKI.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal