Abstract
Background: Patients with sickle cell disease have higher rates of red blood cell (RBC) alloimmunization following transfusion than any other patient population. However, it is unclear if they are inherently more immunizable, or if the high rates of RBC alloimmunization are simply due to antigenic differences between donors/recipients and heightened transfusion frequency. Utilizing a murine model, we have previously shown that rates of alloimmunization are influenced by the inflammatory status of the recipient at the time of the transfusion. Thus, we hypothesized that sickle patients may be more immunizable, due in part to the baseline inflammation associated with their disease. Herein, we present a reductionist murine sickle model of RBC alloimmunization, and investigate the hypothesis that mice with sickle cell disease are more likely to become alloimmunized following transfusion of RBCs containing a foreign antigen than sickle trait controls.
Materials/Methods: Berkeley mice (with human α and βS-globin) were determined to be homozygous (sickle cell disease) or heterozygous (sickle cell trait) by cellulose acetate electrophoresis. We generated the HOD mouse, with RBC specific expression of the model humoral antigen hen egg lysozyme (HEL) fused to the model minor histocompatibility antigen ovalbumin (OVA), linked to the cell membrane by a human blood group antigen (Duffy). The inclusion of OVA in the HOD construct allows presentation of HOD peptides from H-2b MHC; thus, Berkeley mice are able to process and present the HOD antigen. 100 microliters of packed HOD RBCs were transfused IV into mice with sickle cell disease or sickle cell trait; alloimmunization to the HEL antigen was assessed by anti-HEL IgG ELISA 2 weeks following transfusion. Given that co-stimulatory molecule expression on antigen presenting cells in part determines the response of the CD4+ T cell to the antigen being presented, we examined baseline expression of B7-1, B7-2, Ox40L, CD70, 41BBL, CD30L, and CD40 on macrophages (F480 high) and dendritic cells (CD11c high) in sickle cell disease and sickle trait mice.
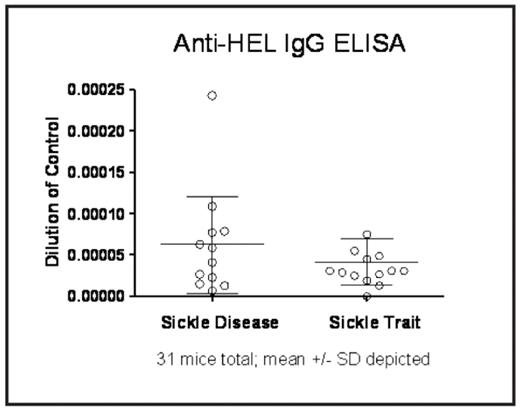
Results: Data for a standard curve was fit to one phase exponential association, with a non-linear best fit regression analysis (R2 of 0.96). Utilizing the OD 415 value of the anti-HEL IgG ELISA, data was combined from 3 separate experiments (n=31 mice). An unpaired two-tailed t-test showed a mean of 6.25e-5, SD 5.87e-5 for sickle disease mice, and a mean of 4.14e-5, SD 2.84e-5 for sickle trait mice, p=0.217 (see figure). Baseline co-stimulatory molecule expression of all molecules examined on macrophages and dendritic cells was similar in both sickle cell disease and sickle trait mice.
Conclusions: Generation of the HOD mouse, containing a model humoral antigen capable of being presented by the antigen presenting cells in the Berkeley mice, allowed these studies to be performed. These data rule out the hypothesis that Berkeley mice with sickle cell disease are substantially more immunizable to a single transfusion of RBCs containing the HOD foreign antigen than are those with sickle cell trait. However, there is a large standard deviation in alloantibody response between individual mice with sickle cell disease. The varied response may be explained by differing levels of illness in individual mice; ongoing studies are investigating such a potential correlation. Clinically, many patients with sickle cell disease are transfused in times of illness (i.e. acute chest syndrome), and the possibility that illness alters alloantibody response cannot be ruled out by these studies. Furthermore, although the model was designed to be as reductionist as possible, these studies are limited by the highly heterogeneous genetic background of the Berkeley mice (including FVB/N, 129, DBA/2, C57BL/6, and Black Swiss); this diverse background likely influences response to foreign antigen and makes identifying control groups difficult. Finally, although Berkeley mice with sickle cell disease are known to have baseline inflammation associated with their disease, this does not appear to influence co-stimulatory molecule expression on their antigen presenting cells.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal