Abstract
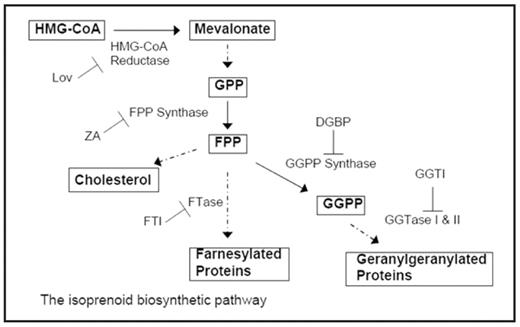
Introduction: The isoprenoid biosynthetic pathway (IBP) is responsible for the production of key sterol and nonsterol species, including farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) which serve as substrates for protein isoprenylation reactions. Several agents known to target the IBP have been observed to have cytotoxic effects in multiple myeloma cells. Thalidomide (Thal) has emerged as an effective agent for treating multiple myeloma. While Thal has been noted to have a variety of direct and indirect effects on myeloma cells, the precise mechanism of action remains unknown.
Aim: We examined interactions between inhibitors of the IBP and Thal in multiple myeloma cells. The mechanisms underlying the observed differential sensitivity to these agents were explored.
Methods: Studies were performed in three human multiple myeloma cell lines (RPMI-8226, U266, H929). Cytotoxicity was assessed via MTT assays, while apoptosis induction was determined by Annexin V staining and evaluation of PARP cleavage. Western blot analysis was used to evaluate inhibition of protein isoprenylation. Intracellular FPP and GGPP levels were measured via enzymatic coupling to fluorescently-tagged peptides, HPLC fractionation and fluorescence detection. Pharmacologic manipulation of the IBP was achieved with the following agents: lovastatin (Lov) as an HMG-CoA reductase inhibitor, zoledronic acid (ZA) as a FPP synthase inhibitor, digeranyl bisphosphonate (DGBP) as a GGPP synthase inhibitor, FTI-277 as a farnesyl transferase inhibitor (FTI), and GGTI-286 as a geranylgeranyl transferase I inhibitor (GGTI).
Results: Addition of Thal to Lov (at both 24 & 48h), zoledronic acid (at 48h), or DGBP (at 24 & 48h) in RPMI-8266 cells results in marked enhancement in cytotoxicity. Isobologram analysis could not be performed as Thal by itself does induce cytotoxicity in MTT assays. Although Lov induces cytotoxicity in a concentration- and time-dependent manner in the U266 and H929 cells, the addition of Thal did not result in increased cytotoxicity. Neither ZA nor DGBP induced cytotoxicity in the U266 cell line, while the H929 cell line showed effects only at 48 hours. Addition of Thal to FTI or GGTI did not result in enhanced cytotoxicity in tested cell lines. Annexin V experiments confirmed enhanced induction of apoptosis in RPMI-8226 cells incubated with the combination of Thal/Lov or Thal/DGBP. Add-back experiments revealed that the enhanced cytotoxicity/induction of apoptosis observed with the addition of Thal could be prevented with the addition of mevalonate or GGPP in Lov-treated cells or GGPP in DGBP-treated cells. PARP cleavage was demonstrated in RPMI-8226 and H929 cells treated with Lov or DGBP (with or without Thal) and in U266 cells treated with Lov. As expected, Lov resulted in the accumulation of unmodified forms of proteins normally farnesylated (Ras) and geranylgeranylated (Rap1a and Rab6) in these cells. Interestingly however, while DGBP led to accumulation of unmodified Rap1a and Rab6 in RPMI-8226 and H929 cells, no effect was seen in the U266 line. Examination of intracellular levels of FPP and GGPP revealed that the U266 line has markedly larger pools of FPP (8.5-fold) and GGPP (2.7-fold) compared to RPMI-8226 and that treatment with DGBP only partially depletes U266 cells of GGPP.
Conclusions: These studies demonstrate an interaction between thalidomide and IBP inhibitors in multiple myeloma cells. These effects appear dependent on depletion of GGPP. Since treatment with a geranylgeranyl transferase-I inhibitor does not produce similar results, this suggests that substrates of geranylgeranyl transferase-II, such as the Rab proteins, may play critical roles in myeloma pathophysiology. The finding that intracellular levels of FPP and GGPP vary markedly amongst cell lines explains differential sensitivity of these cells to pharmacologic manipulation of the IBP and may also influence sensitivity to chemotherapeutic agents. Further studies will determine the extent to which isoprenoid pool sizes vary in primary samples and may ultimately allow for the identification of multiple myeloma patients who would benefit from the addition of an IBP inhibitor to their treatment plan.
Disclosures: Holstein:Celegene: Award. Hohl:Celegene: Consultancy, Speakers Bureau; Terpenoid Therapeutics: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees; Novartis: Consultancy, Speakers Bureau.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal