Abstract
Even with the introduction of rituximab, some patients with lymphoma continue to relapse or progress during treatment. To better define these patients, we looked at all patients with aggressive lymphoma included in the GELA trials during the last 20 years or 4 generations of studies: LNH-87, -93, -98, and -03. Each study generation comprise several randomized studies according to different groups of patients, i.e., young or old, low or high risk. ACVBP, the high-dose regimen used by GELA since 1984 was usually one of the 2 arms, except in elderly patients. Rituximab was first introduced in the LNH-98.5 study and was part of nearly all arms in the LNH-03 study. A total of 7806 patients were included in this retrospective analysis, 3116 being treated with ACVBP and 4880 with other regimens. Two analyses were done: one for the 7198 patients treated without rituximab and one for the 608 patients treated with rituximab. Only patients included in a published study were included explaining the lower number for rituximab-treated patients, most of the LNH-03 studies being not yet published.
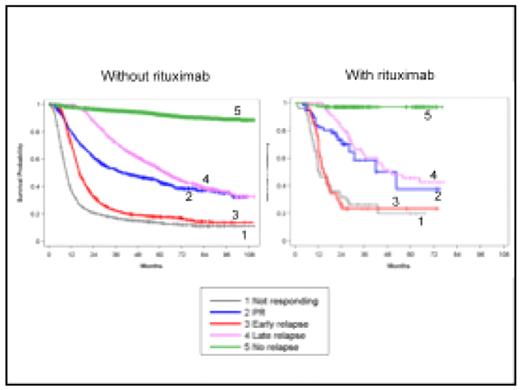
4 groups of patients were defined: refractory or non-responding pts (NR, progression during treatment); patients in PR at the end of treatment (persisting lymphoma cells or PET fixing tumor); relapsing pts with early relapses (ER, during the first year) and late relapses (LR, after one year). 7-year OS was 56% and 7-year PFS was 47.5%, meaning that only 8.5% of the patients were rescued by any treatment at time of progression. Identical results were found in all study generations with 2 groups of patients: those with PR or LR with 7-year OS at 38%, and those with NR or ER, 7-year OS at 12%. Therefore, all generations were grouped (see figure 1). Patients treated with rituximab had a gain of 9% in OS at 6 years (69% vs. 60%). However, they had the same pattern for progression: 6-year OS around 40% for PR and LR patients and around 20% for NR and ER patients. The difference between patients treated with or without rituximab being the percentage of patients in each group: 61% vs. 50% for those without progression and 16% vs. 29% for those with NR or ER. The IPI score does not allow the identification of these poor risk patients. A study is ongoing to characterize the NR+ER patients at diagnosis.
This analysis allowed recognizing 2 patterns of failing therapy. Patients with PR and LR have a disease sensible to chemotherapy while NR and ER pts have a disease not responding to treatment. These patients probably must be treated differently with the introduction of new therapeutic agents into the first line regimen.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal