Abstract
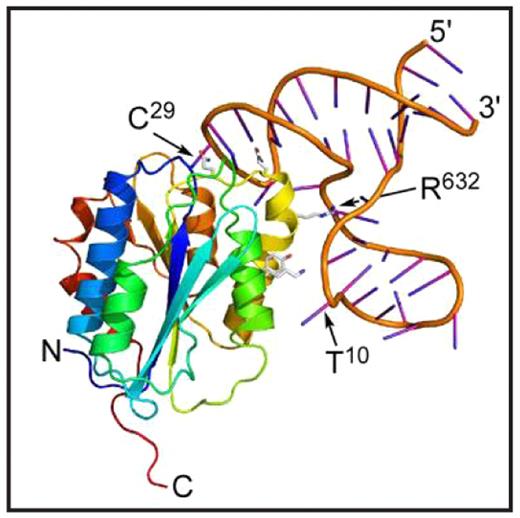
ARC1779 is a modified 40-mer oligonucleotide aptamer that binds with high affinity to the VWF A1 domain and blocks VWF binding to platelet GPIb-alpha. When given intravenously to volunteers and assayed ex vivo, ARC1779 dose-dependently inhibits VWF activity and prolongs the PFA-100 closure time with EC90 values of 2–3 μg/ml. ARC1779 also inhibits carotid artery thrombosis induced by electrical injury in nonhuman primates. These properties suggest that ARC1779 may be useful to prevent or treat platelet-dependent thrombosis in acute coronary syndromes, percutaneous coronary intervention procedures, or thrombotic thrombocytopenic purpura. To characterize this aptamer-VWF interaction, we determined the crystal structure of a complex between recombinant VWF domain A1 (residues 497–705, mature subunit numbering) expressed in E. coli and ARC1172, a 41-mer DNA oligonucleotide with the sequence 5′-GGC GTG CAG TGC CTT CGG CCG TGC GGT GCC TCC GTC ACG CC-invdT-3′. ARC1779 was derived from ARC1172 by adding modifications to prevent digestion by nucleases, and both aptamers bind VWF A1 with the same affinity. Crystals of the complex belonged to space group P212121 and diffracted to 2.4 angstroms. The A1 domain (Figure) has essentially the same structure as observed previously in isolation or bound to GPIb-alpha, botrocetin, bitiscetin, or Fab NMC4. Therefore, aptamer binding does not substantially alter the conformation of the VWF A1 domain. ARC1172 consists of three short helical segments and three connecting loops. Nucleotides C32-C40 interact with two segments G23-G26 and G2-G6 to form a 9-basepair helix with an interruption in one strand. The interruption comprises residues C7-T22, which protrude to form a 3-basepair helix between G11-C13 and G17-C19. The loop of ARC1172 composed of nucleotides G26-C32 makes extensive contacts with VWF A1 residues in helix alpha-4 (yellow) and alpha-5 (orange) and loop betaD-alpha4. For example, the side chains (shown in Figure) of Q625, Q628, R632 and Y637 participate in numerous hydrogen bonds and salt-bridges with ARC1172 (e.g., Q625 contacts C29), and R632 is buried within a deep pocket of the aptamer. Botrocetin occupies a similar interface on VWF A1, which accounts for the ability of ARC1172 or ARC1779 to block botrocetininduced platelet aggregation. In addition, nucleotide T10 extends toward helix alpha3 (lime green) and obstructs residue K599, which is required for binding to platelet GPIbalpha. The results provide a structural explanation for many of the functional properties of aptamers ARC1172 and ARC1779.
Disclosures: Diener:Novartis Institute for BioMedical Research: Employment; Archemix Corp.: Equity Ownership. Schaub:Archemix Corp.: Employment, Equity Ownership. Sadler:Baxter Biosciences: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Ablynx: Consultancy.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal