Abstract
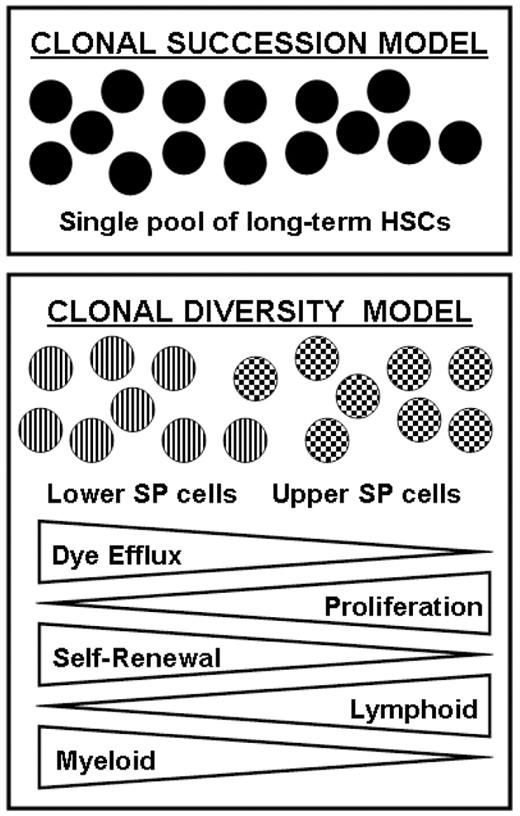
Over the decades since hematopoietic stem cells (HSCs) were first identified, the traditional view has been that the hematopoietic system is regenerated by a single pool of multipotent, quiescent HSCs that are sequentially recruited into cell cycle and which then progressively divide and differentiate until they are exhausted and ultimately replaced by the next cohort of stem cells. However, recent evidence has challenged this classical clonal succession model of HSC hierarchy by suggesting that the hematopoietic system is maintained by a pool of different HSC subtypes, with distinct self-renewal and differentiation potentials (the clonal diversity model, Figure 1). The side population (SP), characterized by Hoechst dye efflux, has been used as a method for isolating HSCs for over a decade and the SP has been shown to be highly enriched for HSC activity. While the entire SP is strikingly homogeneous with respect to expression of canonical stem cell markers such as Sca-1 and c-Kit, we recently observed heterogeneous expression for the SLAM family molecule CD150 within the SP, with CD150+ cells more prevalent in the lower SP and CD150− cell more prevalent in the upper SP. We decided to examine this observation further by investigating the properties of cells from different regions of the SP. Functional capacity was assessed by competitive bone marrow transplantation of upper SP cells, lower SP cells, and a combination of the two populations. Lower SP cells showed better engraftment than upper SP cells in recipient mice, a trend that continued when donor HSCs were isolated from primary recipients and re-transplanted into secondary hosts. Lower SP cells showed 3-fold better engraftment than upper SP cells in secondary transplants, suggesting better self-renewal capacity. However, analysis of the hematopoietic lineages formed by donor cells in recipient mice demonstrated that while both upper and lower SP cells were capable of forming all mature lineages, lower SP cells were biased towards myeloid differentiation while upper SP cells were biased towards lymphoid differentiation. The lineage biases observed from transplantation of one cell population alone were exacerbated when both upper and lower SP cells were co-transplanted into the same recipient mouse, suggesting that while both populations are capable of forming all hematopoietic lineages, in the presence of the other stem cell type (as would be the case in normal homeostasis) that the majority of the output from each HSC subtype is almost exclusively lymphoid or myeloid. The lineage contribution trends observed in the peripheral blood were also reproduced when bone marrow of transplanted mice was analyzed, including at the level of progenitors with lower SP cells showing greater ability to make myeloid progenitors (megakaryocyte-erythrocyte progenitors and granulocyte-macrophage progenitors) and upper SP cells producing proportionately more common lymphoid progenitors. Microarray analysis of upper and lower SP cells to determine the molecular signatures underlying these functional differences found many genes critical for long-term HSC self-renewal to be highly expressed in lower SP cells including Rb1, Meis1, Pbx1 and TGFbr2 while upper SP cells showed higher expression of cell cycle and activation genes. Cell cycle analysis showed upper SP cells to be approximately 2-fold more proliferative than lower SP cells (18.9% to 8.3% Ki-67+, 39.4% to 20.1% BrdU+ 3-days post-BrdU administration). The clonal diversity model which proposes the adult HSC compartment consists of a fixed number of different HSC subtypes each with pre-programmed behavior has important implications for using HSCs in experimental and clinical settings. While other studies have provided functional evidence for the clonal diversity model, this is the first study to prospectively isolate the functionally distinct HSC subtypes prior to transplantation.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal