Abstract
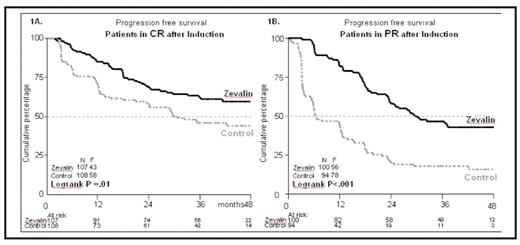
Initial findings from the Phase 3 trial of Zevalin consolidation of first remission in patients with advanced follicular lymphoma showed a significant improvement in PFS following Zevalin consolidation as compared to no further treatment, ie, 37.5 vs. 13 months (Hagenbeek et al, ASH 2007). Patients had been randomised to either no further therapy or Zevalin consolidation (250 mg/m2 rituximab on day -7 and on day 0, followed on day 0 by Zevalin 0.4 mCi/kg; maximal dose 32 mCi). Herein we report on an additional 1-year follow-up of the FIT study, at a median of 42 months (range: 2 – 77), which includes 409 patients who achieved a complete or partial response (CR or PR), after induction therapy. Patient demographics remain similar in the Zevalin (n=202) and control groups (n= 207): males 48%/50%; median age 55/53 yrs; at diagnosis, stage III 35.2%/31%, stage IV 64%/66%. Response status prior to randomization was also similar between study arms: 107 patients in CR/CRu and 100 pts in PR in the Zevalin arm and 108 pts in CR and 88 pts in PR in the control arm. The 48-month PFS is 52% in the Zevalin arm compared with 31% in the control arm. As shown in the figure 1A, patients with a CR after induction therapy achieved a median PFS of >67 months for the Zevalin arm compared with 30.8 months for patients who received no further treatment (HR 0.61 [95% CI .41–.91]; P= 0.015). In figure 1B, among patients with a PR after induction, the median PFS was 29.6 months for those on the Zevalin arm vs 6.7 months for those on the control arm (HR 0.36 [95% CI .25–.51]; P<0.001). Subsequent therapy with various modalities including chemotherapy, radiotherapy, immunotherapy, Zevalin and stem cell transplantation (ASCT) was given to 63 patients in the Zevalin arm and 108 patients in the control arm, who achieved an overall response rate of 81% and 73%, respectively. A total of 11 deaths have occurred in the Zevalin arm and 8 in the control arm, with no difference in overall survival between the treatment arms (P=0.593). No additional toxicities, no congenital malformations, and no increase in the incidence of secondary malignancies have emerged during follow-up. Details on subsequent therapies and a breakdown of outcomes based on induction subgroups and FLIPI scores are anticipated for presentation.
Conclusion: Extended follow-up of the FIT trial shows that Zevalin consolidation does not preclude the use of various effective second-line treatment options. Moreover, these data confirm that the significant improvement in PFS achieved with Zevalin consolidation is durable for both patients with a PR and patients with a CR/CRu after induction.
Disclosures: Morschhauser:Bayer Schering: Honoraria; Roche: Honoraria. Bischof-Delaloye:Bayer Schering: Consultancy, Honoraria. Rohatiner:Bayer Schering: Research Funding. Salles:Bayer Schering: Honoraria; Roche: Honoraria. Kuhlmann:Bayer Schering: Employment. Soubeyran:Bayer Schering: Consultancy, Honoraria. Van Hoof:Bayer Schering: Honoraria. Hagenbeek:Bayer Schering: Consultancy, Honoraria; Roche: Consultancy, Honoraria.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal