Abstract
Background: The plasma cell labeling index (LI), a slide-based measure of the percentage of bone marrow plasma cells in the S-phase of cell cycle, is a powerful prognostic tool in multiple myeloma (MM). A high LI is a marker of poor outcome, and is a useful adjunct to cytogenetics and fluorescent in-situ hybridization to risk-stratify MM patients. It has been shown to provide additional prognostic information in International Staging System (ISS) stages 1 and 2 patients treated with conventional chemotherapy ± autologous stem cell transplantation. However, the impact of a high LI is not known in patients receiving novel agents that have been integrated into the current treatment paradigm of newly diagnosed MM. The objective of this study was to determine the value of LI in patients treated with thalidomide-dexamethasone (Thal/Dex) or lenalidomide-dexamethasone (Len/Dex).
Patients and Methods: Data from 125 newly diagnosed MM patients enrolled in clinical trials with either thalidomide (200mg/d) plus dexamethasone (Thal-Dex; n=50), or lenalidomide (25 mg/d on days 1–21 of a 4-week cycle) plus dexamethasone (Len-Dex; n=75) as initial therapy, were utilized. Patients within both the studies were categorized into 2 LI groups based on baseline LI: high (≥1%) vs. low (<1%) LI. Overall survival (OS) and progression-free survival (PFS) were estimated using Kaplan Meier method, and compared between the two LI groups within the Thal Dex and Len-Dex studies using log rank tests.
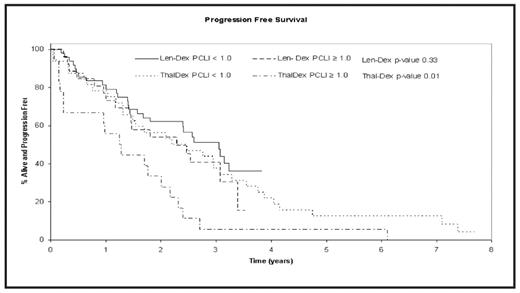
Results: The median follow-up for patients on Thal-Dex and Len-Dex was 7.5 and 3 years, respectively. 36% (18/50) of patients in the Thal-Dex study and 35% (26/75) in the Len-Dex study had high LI. The baseline patient-characteristics, including age, stage (ISS), lactate dehydrogenase, calcium, hemoglobin and creatinine were comparable between the 2 studies. The median OS for patients receiving Thal-Dex was 4.7 years (95% CI 3.1–8.1); 2.3 years (95% CI 1.3–4.6) for patients with LI ≥1 compared to 6.9 years (95% CI: 3.9–NA) for those with LI <1 (P=0.02). The median OS for patients on Len-Dex was not reached for either the high or low LI group (P=0.80). The median PFS for all Thal-Dex patients was 1.7 years (95% CI: 1.4–3.1); 1.3 years (95% CI: 0.2–2.0) for patients with high LI vs.2.3 years (95% CI: 1.3–3.3) for patients with low LI; P = 0.01. The median PFS for Len-Dex patients was 2.6 years (95% CI: 1.8–3.1) years, and no significant difference was observed between the 2 LI groups, with PFS of 2.3 years (95% CI: 1.4–3.1) for patients with high LI, and 3.1 years for patients in the low LI group. Due to a shorter median follow-up of Len-Dex patients, a separate analysis censoring all patients in both the studies at 3 years was performed with similar results.
Conclusion: LI remains a significant determinant of PFS and OS in newly diagnosed MM patients treated with Thal-Dex, with high baseline LI predicting poorer outcome. In contrast, during first 3 years of follow-up, the unfavorable prognostic effect of LI is not apparent with Len-Dex therapy, and longer follow-up is needed to determine if Len-Dex therapy overcomes the poor prognostic effect of high LI.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal