Abstract
Clinical trials in patients with hemophilia B have demonstrated considerable inter-patient variability in the pharmacokinetics (PK) of Factor IX (FIX) replacement therapy, including the recovery, an important PK parameter from which individualized clinical dosing decisions are calculated. In clinical trials of plasma-derived and recombinant factor IX replacement therapies, the age of the patient has been demonstrated to affect recovery (younger patients have lower recovery values than older patients), however the specific contribution of age, as well as additional covariates such as body weight and race to PK variability has not been systematically evaluated. We analyzed an extensive database of BeneFIX PK data collected from 8 separate clinical trials conducted over 13 years. A systematic approach involving population PK modeling and simulation was utilized for the first time to estimate the effects of individual-specific covariate factors on PK of BeneFIX in the pooled population that included pediatric and adult patients. A total of 4025 plasma FIX activity PK data sets collected from 191 patients, aged 0 to 69 years were used for the analysis. There were 111 children (£15 years) including 53 infants <2 years, and 80 adults (>15 years) in the pooled data. The majority (84%) of patients were Caucasian. The remaining patients were African American (7%), Hispanic (4%), Asian/Japanese (3%), and other ethnicity (3%).
The data were analyzed using nonlinear mixed-effects modeling with the NONMEM software system. Age, weight, and race were examined as covariates for the ability to explain inter-individual variability in the BeneFIX PK. The PK in pediatric and adult patients was described by a two-compartment model with first-order elimination and a zero-order input using the following parameters: clearance (CL), volume of central compartment (V1), volume of peripheral compartment (V2) and inter-compartmental clearance (Q). Population predicted BeneFIX PK parameters, standardized to a 70 kg patient, were 7.46 (standard error; 0.20) mL/hr/kg, 131 (4.4) mL/kg, 71.5 (2.1) mL/kg and 12.1 (1.1) mL/h/kg, for CL, V1, V2 and Q, respectively. The final model was able to simulate data in close agreement with the actual study observations.
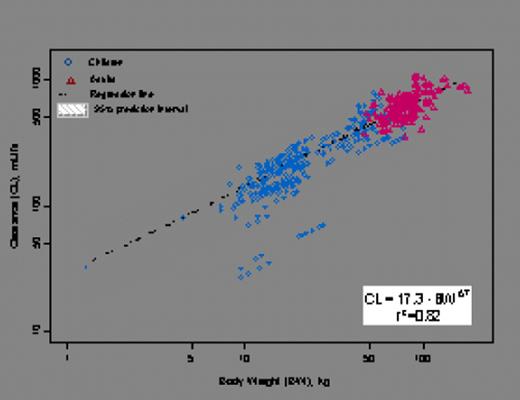
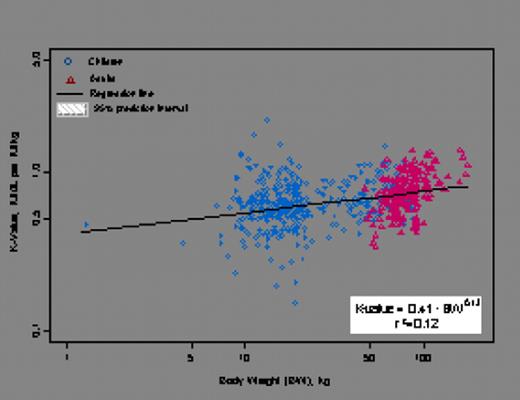
Variability (%CV) in BeneFIX PK was explained most significantly by allometrically scaled body weight (Figure 1a), while age and race had no discernible effects on BeneFIX PK in the population studied. Observed recovery values were slightly lower in children (£15 years) compared with those in adults (>15 years) since the initial volume of distribution (V1), normalized to body weight, was slightly higher in children than in adults, while the variability in the observed recovery values was comparable between children and adults (Figure 1b).
In conclusion, the present analysis, for the first time, systematically describes and quantifies the sources of age-dependent variability of factor IX PK, using BeneFIX data, and provides a better understanding of the importance of body weight in the disposition of BeneFIX. This confirms existing weight-based dosing recommendations and further supports consideration of dosing adjustments that are individualized based on the patient’s body weight in the context of the achieving the desired clinical response, such as recovery. This also may be important in pediatric patients during growth periods associated with significant weight change.
Clearance versus Body weight
Recovery versus Body weight
Disclosures: Udata:Wyeth Research: Employment. Sullivan:Wyeth Research: Employment. Kelly:Wyeth Research: Employment. Roth:Wyeth Research: Employment. Meng:Wyeth Research: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal