Abstract
INTRODUCTION: Acute myeloid leukemia (AML) is a heterogeneous disease with varying survival rates depending mostly upon the molecular phenotype of the single leukemic clone. The most powerful predictor for the outcome of the individual patient is the cytogenetic profile at the time of diagnosis, dividing the patients into good, intermediate and adverse prognostic group. However, given that 40–60 percent of patients exhibits a normal karyotype and are assigned to an intermediate prognostic group, identification of biologic parameters, which either alone or in combination, predict disease outcome more precisely are needed. We have previously performed a gene expression profiling study (Grubach et al, Eur J. Hematol. 2008 Apr 10. [Epub ahead of print]) on a series of Polycomb, Hox and Meis genes expressed in hematopoietic cells. AIM: Based on the finding that HOXA4 could be used as a predictor for outcome in AML patients with a normal karyotype, we hypothesized that combining the gene expression of the HOXA4 gene and co-factor MEIS1 might unravel a leukemogenic impact in other cytogenetic prognostic groups (Grimwade et al. Blood. 1998 Oct 1;92(7):2322–33). In addition, given that epigenetic events might contribute to the regulation of these genes, we determined whether promoter hypermethylation of CpG islands in the promoter regions were of relevance to the expression levels of HOXA4 and MEIS1.
MATERIALS & METHODS: Diagnosis samples from 248 AML patients were analyzed by RQ-PCR for expression levels of HOXA4 and MEIS1. 157 of these patients were further analyzed for promoter hypermethylation of the same genes by bisulphite treatment of DNA followed by methylation-specific melting curve analysis (MS-MCA).
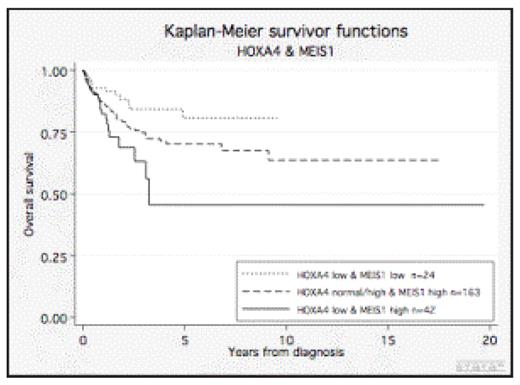
RESULTS: When combining the gene expression levels of HOXA4 with MEIS1 into the three main groups (low HOXA4/low MEIS1, low HOXA4/high MEIS1 and normal-high HOXA4/high MEIS1; (the latter pooled to enable statistical calculations)), clear differences in overall survival were found (Fig. 1). Thus, within the group of patients exhibiting low levels of HOXA4 transcript, those with a high expression of MEIS1 had a significantly worse outcome than those having low MEIS1 expression (p=0.025). Importantly, in a multiparameter regression analysis, the prediction was independent of the cytogenetic grouping, of mutations in NPM1 and FLT3 genes, WBC and age. Given the efficacy of demethylating therapy, we also considered the mechanism of HOXA4 and MEIS1 gene regulation. Thus, when promoter methylation of HOXA4 and MEIS1 in 157 patients was investigated, we found that 15 % of the patients had hypermethylation of the promoter region of MEIS1 and 77% of the patients showed hypermethylation of HOXA4. Importantly, a significant correlation for both of the genes between the expression level and methylation state was observed (MEIS1, p=0.001 and HOXA4, p=0.007).
CONCLUSION: The altered expression levels of HOXA4 and MEIS1 in AML reflect, at least partly, an epigenetic regulation by virtue of promoter hypermethylation. The level of transcripts of HOXA4 and MEIS1 seem to contribute to the leukemogenesis in AML and can serve as independent prognostic variables regardless of their cytogenetic and molecular background.
Overall survival of AML patients-stratified by cytogenetics, mutations in NPM1 and FLT3, WBC and age. By combination of HOXA4 and Meis1 expression a significant better survival is linked to those with a low HOXA4/low MEIS1 compared to those with a low HOXA4/high MEIS1 expression.
Overall survival of AML patients-stratified by cytogenetics, mutations in NPM1 and FLT3, WBC and age. By combination of HOXA4 and Meis1 expression a significant better survival is linked to those with a low HOXA4/low MEIS1 compared to those with a low HOXA4/high MEIS1 expression.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal