Abstract
Background: Although GVHD prophylaxis without MTX might enhance a graft-versus-leukemia effect, no randomized controlled trial (RCT) of GVHD prophylaxis has investigated this possibility in a reduced-intensity stem cell transplantation (RIST) setting. Therefore, we conducted a prospective randomized trial to compare CSP and TAC without MTX as GVHD prophylaxis in RIST from a MRD.
Methods: Patients with hematological malignancies in complete remission or with a chemosensitive status were eligible for this study if they were either older than 50 years or had significant medical contraindications for conventional transplantation. The primary endpoint was the incidence of grade II-IV acute GVHD on day 100. Regimen-related toxicities (RRT) between day −8 and day 28 were assessed by NCI-CTC ver 2.0. The conditioning regimen consisted of cladribine (0.11 mg/kg × 6 days) and oral busulfan (4 mg/kg × 2 days). All patients received unmanipulated G-CSF-mobilized peripheral blood stem cells from a MRD. CSP (starting dose 3 mg/kg/day, target whole blood conc. 250–350 ng/mL, target trough level 150–250 ng/mL) or TAC (starting dose 0.03 mg/kg/day, target whole blood conc. 10–20 ng/mL, target trough level 5–10 ng/mL) was given as GVHD prophylaxis from day −1. G-CSF was administered from day +6 until neutrophil engraftment.
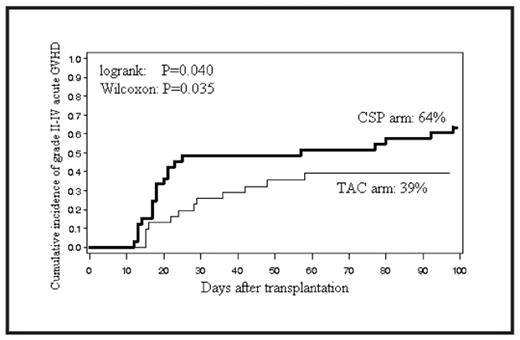
Results: Sixty-eight patients were enrolled between 2/2003 and 2/2008, but 3 were removed before transplant because of disease progression or infection. The diagnoses included lymphoma (n=27; 10 FL, 6 DLBCL, 6 PTCL and 5 HL), AML (n=14), MDS (n=12), MM (n=5), MPD (n=4) and ALL (n=3). The median age of the patients (56 y vs. 55 y) and the median number of CD34+ cells infused (3.7 ×106/kg vs. 3.1 ×106/kg) were similar in the CSP and TAC arms. The median day of neutrophil engraftment in both arms was day 11. The proportion of patients in the CSP or TAC arm who achieved complete chimerism in CD3+ cell fraction on days 28, 56 and 90 was, respectively, 61% vs. 48%, 80% vs. 76% and 80% vs. 97%. Grade 4 RRT was hepatic disease (n=1, CSP arm), and grade 3 RRT included cardiac (n=1, CSP), renal (n=1, CSP), hepatic (n=2, CSP), oral mucosal (n=1, TAC) and gastrointestinal disease (n=4, CSP vs. n=3, TAC). The incidence of grade II–IV acute GVHD in the TAC arm was significantly lower than that in the CSP arm (Table & Figure). The incidences of grade III–IV acute GVHD and extensive chronic GVHD were not significantly different between the two arms. The non-relapse mortality (NRM) in the TAC arm was significantly lower than that in the CSP arm. The causes of death that contributed to NRM were infection in 6 (CSP arm), GVHD in one (CSP), ARDS in one (TAC) and lung cancer in one (CSP). The relapse rate and relapse-related mortality were not significantly different between the two arms. The median follow-up for surviving patients was 1295 days (169–1954). The overall survival (OS) and progression-free survival (PFS) rates in the TAC arm tended to be higher than those in the CSP arm.
Conclusions: A regimen with TAC alone without MTX as GVHD prophylaxis was associated with significantly lower rates of grade II–IV acute GVHD and NRM compared to a regimen with CSP alone after RIST from a MRD. OS and PFS with TAC alone tended to be higher than those with CSP alone. Nevertheless, these results must be considered with care due to the small number of patients, and the optimal use of both drugs is still under investigation. A large-scale RCT to identify suitable GVHD prophylaxis in the RIST setting is warranted.
Table: Study Outcomes
| . | CSP arm (n=33) . | TAC arm (n=32) . | P . |
|---|---|---|---|
| P value was evaluated with logrank test or Wilcoxon test*. | |||
| Grade II–IV acute GVHD | 64% | 39% | 0.040 |
| Grade III–IV acute GVHD | 30% | 23% | N.S |
| Extensive chronic GVHD | 63% | 61% | N.S |
| 1-year/3-year NRM | 26%/38% | 0%/5% | 0.008 |
| 1-year/3-year relapse | 35%/43% | 25%/49% | N.S |
| 1-year/3-year relapse mortality | 27%/33% | 10%/42% | N.S |
| 1-year/3-year OS | 57%/45% | 90%/56% | 0.135 (0.039*) |
| 1-year/3-year PFS | 48%/35% | 75%/48% | 0.120 (0.047*) |
| . | CSP arm (n=33) . | TAC arm (n=32) . | P . |
|---|---|---|---|
| P value was evaluated with logrank test or Wilcoxon test*. | |||
| Grade II–IV acute GVHD | 64% | 39% | 0.040 |
| Grade III–IV acute GVHD | 30% | 23% | N.S |
| Extensive chronic GVHD | 63% | 61% | N.S |
| 1-year/3-year NRM | 26%/38% | 0%/5% | 0.008 |
| 1-year/3-year relapse | 35%/43% | 25%/49% | N.S |
| 1-year/3-year relapse mortality | 27%/33% | 10%/42% | N.S |
| 1-year/3-year OS | 57%/45% | 90%/56% | 0.135 (0.039*) |
| 1-year/3-year PFS | 48%/35% | 75%/48% | 0.120 (0.047*) |
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal