Abstract
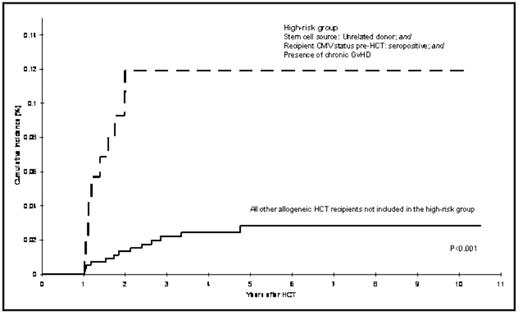
Patients undergoing HCT are at an increased risk of developing primary and/or reactivated CMV infection, although the magnitude of risk of CMV disease has decreased with the widespread use of preemptive ganciclovir. Most episodes of reactivation occur within the first year post-HCT and are associated with risk factors such as CMV serostatus of donor and recipient, development of acute graft vs. host disease (GVHD): and the immunosuppressive therapy used for its management. Because of prolonged periods of immunosuppression post-HCT, patients may be at risk for delayed CMV infection one or more years after HCT. However, the magnitude of risk of delayed CMV infection and characteristics of those at increased risk has not been described. Given the high morbidity and mortality associated with post-HCT CMV infection, identifying patients at high risk of delayed CMV could be useful for effective management. This report includes 2700 consecutive patients who survived more than one year after undergoing HCT at COH between 1976 and 2003; these included 1404 autologous HCT recipients and 1296 allogeneic HCT recipients (1043 related donor; 253 unrelated donor recipients). Median age at HCT was 38 years (range, 0.6 to 79 years) and 59% of the cohort was males. Median follow-up time from HCT until delayed CMV infection/disease, death, or end of study period (12/31/2006), whichever occurred first, was 4.3 years (range:1–26.6 years). Medical records from COH and/or outside facilities were the main source of data for CMV occurrences. In total, 33 patients (1%) developed delayed CMV infection after surviving at least one year post-HCT (1 autologous and 32 allogeneic [20 related donor and 12 unrelated donor HCT]) developed a total of 40 episodes of delayed CMV that included pneumonia (n=16), gastrointestinal disease (n=8), retinitis (n=2), hepatitis (n=1), concurrent pneumonia and hepatitis (n=1), and asymptomatic reactivation (n=12). The overall cumulative incidence of delayed CMV infection was 1.3% (95% Confidence Interval [CI], 0.9–1.8%) at 5 years from HCT. For autologous HCT recipients, the incidence was 0.07% at 1 year based on 1 event. Among allogeneic HCT recipients, the cumulative incidence at 5 years post-HCT was 2.1% [95%CI, 1.2–3.0%] for related donor HCT recipients; and 5.0% [95%CI, 2.2–7.7%] for unrelated donor HCT recipients. Among allogeneic HCT recipients, the risk factors for the development of delayed CMV infection included unrelated donor HCT (hazard ratio [HR] = 2.5, 95% CI, 1.1–5.7) and CMV seropositive status of the recipient (HR=7.7, 95% CI 1.0–57.0) (Figure). Interestingly, donor CMV status was not associated with increased risk of delayed CMV. All 32 allogeneic HCT recipients experienced chronic GVHD, with prolonged exposures to corticosteroids (median=494 days), and cyclosporine (median=380 days). Thirty patients with delayed CMV infection (94% of the allogeneic HCT recipients with delayed CMV) were receiving immunosuppressive therapy for management of chronic GVHD at onset of delayed CMV. A total of eight patients with delayed CMV did not have a history of CMV infection in the first year, and were characterized by the following clinical and demographic features: 6 (75%) were male; median age at HCT was 35 years; one was an autologous HCT recipient, who relapsed 10 months post-HCT for non-Hodgkin lymphoma, received further chemotherapy and radiation, including Rituximab and then developed late CMV, just over one year post-HCT. The seven allogeneic HCT recipients had chronic GVHD, and were CMV serostatus positive prior to HCT, with 4 also having CMV seropositive donors. Of the 33 patients with delayed CMV in this study, 26 expired; median survival after the development of delayed CMV was 46 days. This study describes the magnitude of risk of delayed CMV infection in autologous and allogeneic HCT recipients and identifies at risk patients as those who are seropositive for CMV, undergoing unrelated HCT, and those with prolonged exposures to immunosuppressive therapy for cGVHD (Figure), suggesting the need for a close surveillance of these patients at high risk.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal