Abstract
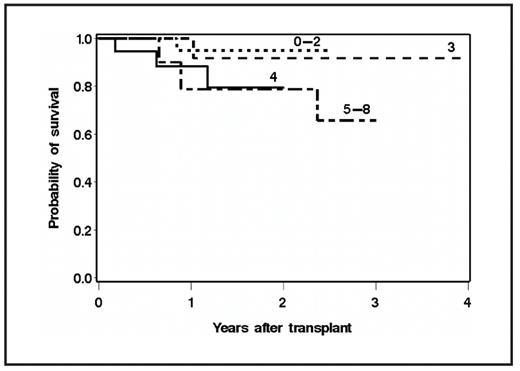
Mantle Cell Lymphoma (MCL) exhibits short remission durations and a poor prognosis. To improve on these outcomes, many have advocated the use of high-dose therapy (HDT) and ASCT. The MIPI predicts overall survival (OS) from diagnosis, yet it remains unknown if the MIPI assessed at diagnosis (MIPI-Dx) or prior to transplant (MIPI-Tx) can be used to predict OS from ASCT. To address this question we retrospectively evaluated the association of the MIPI-Dx and MIPI-Tx, and other characteristics with OS following HDT and ASCT in 87 consecutive MCL patients transplanted at our center. Baseline characteristics included: median age at diagnosis=56 years (range, 35–70), median age at transplant=57 years (range, 35 – 70), stage III-IV=97%, median LDH/upper limit of normal (ULN) at diagnosis=0.91 (range, 0.46–5.65), median LDH/ULN at transplant= 0.88 (range, 0.39–3.00), median white blood cell (WBC) count at diagnosis=7.50 x 109 / liter (range, 1.40 – 54.70), mean WBC count at transplant=4.66 x 109 /liter (range, 0.07 – 17.60), median number of prior chemotherapy regimens=2 (range, 1–5). The estimated 5-year OS and PFS for the entire cohort were 56% (95% CI, 39–73%) and 45% (95% CI, 30 – 60%), respectively with a median follow up among surviving patients of 2.0 years (range 0.1 – 10.1 years). The MIPI-Dx was a better predictor of OS (p<0.001) than MIPI-Tx (p=0.09), when evaluated as a continuous variable. Similarly, when stratified as a categorical variable, the MIPI-Dx (p=0.09) was superior to the MIPI-Tx (p=0.34) in estimating survival. When compared to patients with a MIPI-Dx of 0–2, those with a score of 3, 4, and 5–8 at diagnosis had a 3.3 (95% CI 0.3–32, p=0.3), 6.1 (95% CI 0.7–54.8, p=0.11), and 11.1 (95% CI 1.3–92.9, p=0.03) fold higher risk of mortality after transplant, respectively (Figure). We next determined if any pre-transplant factors could improve our ability to predict outcome after ASCT. Multivariable modeling identified pretransplant factors of ECOG PS >0 (hazard ratio (HR) of 3.0, p=0.03), number of prior regimens >2 (HR 5.2, p=0.01), lack of complete remission (CR) (HR 3.4, p=0.04), and elevated LDH (HR 4.4, p=0.01) as associated with higher risk of death after transplant. These results indicate that the MIPI-Dx is a robust prognostic tool that can even be used to predict outcomes after a later transplant, suggesting that it may continue to reflect the biology of an individual patient’s disease over time. Further assessment of survival predictions after ASCT can be made independently by examining pre-transplant factors including performance status, number of prior regimens, attainment of CR, and LDH. Though these data require prospective validation, our results can be used to counsel patients about outcomes and account for potential differences in results from clinical trials of HDT and ASCT for MCL.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal