Abstract
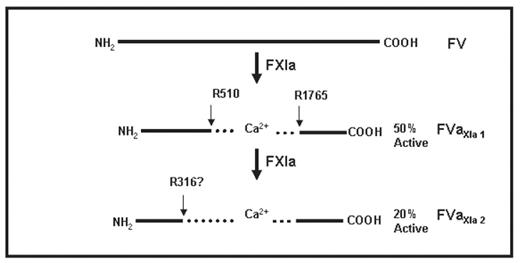
The procofactors FV and FVIII are activated by thrombin, FXa and plasmin. During contact pathway-initiated thrombin generation, FXIa activates FIX thus feeding into the coagulation cascade; however, the procofactors FVIII and FV must be activated to achieve a robust level of thrombin generation. We tested the hypothesis that FXIa can activate FV and FVIII. FV (1uM) was subjected to FXIa (100nM) proteolysis. During the reaction the relative activity and integrity were measured at selected time points using a one stage PT clotting assay and SDS-PAGE. Over the 60 minute time course, FV showed a transient 50% activation followed by a reduction in activity to 20%. SDS-PAGE analyses showed that proteolysis of FV by FXIa initially generated fragments with mobilities similar to those produced by α-thrombin. This activation and inactivation pattern suggested that FXIa makes the required activation cleavages at R709, R1018 and R1545 coincidently with inactivating proteolyses. FV was activated with α-thrombin and the reaction quenched by the addition of hirudin prior to FXIa proteolysis. FVa’s cofactor activity was reduced by 50% after 30 min and 80% after 60 min. Analysis of the FXIa cleavage process by SDS-PAGE under reducing conditions showed no intact heavy chain and significant proteolysis of the light chain after 30 minutes. In addition to the Mr = 105000 (HC) and Mr = 75000 (LC), six new products were identified by SDS-PAGE under reducing conditions: Mr = 54000, 50000, 48000, 30000, 22000 and 20000. NH2-terminal sequence analysis indicated a single cleavage in the light chain at R1765 yielding the products Mr = 50000, 48000: (1766à2196) and the Mr = 30000 (1546à1764), also seen with plasmin and FXa. A cleavage in the heavy chain represented by the Mr = 22000, 20000 (R511à709) fragments is also observed. Sequence analysis determined that the Mr = 54000 fragment represented the NH2-terminus of the heavy chain. Western analysis using a heavy chain antibody showed a transient band Mr = 75000 lying under the light chain that is consistent with an initial cleavage R510. A subsequent cleavage, which is coincident with a decrease in the cofactor’s activity, results in a Mr = 30000 product which is consistent with a cleavage somewhere COOH-terminal to R306(R316?). Activity analyses suggest that the initial cleavage in the heavy chain at R510 leads to a FVaXla 1 molecule with similar activity (50%) to that seen in FVa cleaved by APC in the absence of phospholipid. The FVaXla 1 was treated with APC resulting in complete inactivation of the cofactor. We have also observed an analogous FXIa cleavage pattern in FVIII; however sequencing analysis has not yet been attempted. These data suggest that factor XIa may play a role in procofactor activation and inactivation of FV and FVIII in the context of contact pathway-initiated blood coagulation.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal