To the editor:
With great interest, we have read the article by Beers et al1 documenting a potent B-cell depleting ability for type II (or tositumumab-like) CD20 antibodies. The authors conclude that complement-dependent cytotoxicity (CDC) is of little importance for B-cell depletion induced not only by type II, but also by type I (or rituximab-like) CD20 antibodies. Unfortunately, the experimental design chosen by Beers et al does not allow such a conclusion, and the data in our view appear to suggest the reverse.
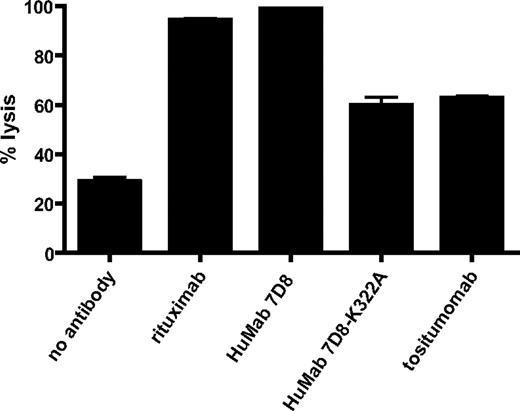
First, Beers et al show that tositumomab also induces CDC, albeit at a lower level than rituximab. Similarly, we also routinely observe significant complement-mediated lysis of lymphoma cells by tositumomab (Figure 1). Second, to specifically address the role of complement, the authors generated a mouse IgG2a version of rituximab (Rit-m2a) and introduced a lysine-into-alanine mutation at position 322 (K322A) to abrogate C1q binding and CDC. A study by Idusogie et al,2 however, previously demonstrated this mutation to be insufficient to remove CDC activity, and K322A-mutated rituximab retained more than 60% of its CDC capacity at near-physiologic complement levels. Indeed, we have confirmed that for other CD20 antibodies, such as HuMab 7D8,3 significant CDC activity remains after mutating K322 (Figure 1). Hence, on basis of their data, Beers et al cannot exclude complement activation as an in vivo mechanism of action for B-cell depletion by either tositumomab or rituximab.
Complement-dependent cytotoxicity of CD20 antibodies. Daudi cells (0.1 × 106) were incubated with CD20 antibodies rituximab, HuMab 7D8, HuMab 7D8-K322A or tositumomab (10 μg/mL) at room temperature for 15 minutes. Normal human serum (final concentration 20%) was added as a source of complement, and cells were incubated for 45 minutes at 37°C. Propidium iodide was added, and cells were analyzed by flow cytometry. Results are shown as percentage of propidium iodide–positive cells proportional to total cell number (% lysis) and are representative of 3 separate experiments.
Complement-dependent cytotoxicity of CD20 antibodies. Daudi cells (0.1 × 106) were incubated with CD20 antibodies rituximab, HuMab 7D8, HuMab 7D8-K322A or tositumomab (10 μg/mL) at room temperature for 15 minutes. Normal human serum (final concentration 20%) was added as a source of complement, and cells were incubated for 45 minutes at 37°C. Propidium iodide was added, and cells were analyzed by flow cytometry. Results are shown as percentage of propidium iodide–positive cells proportional to total cell number (% lysis) and are representative of 3 separate experiments.
Notably, the authors point out that it is not understood why the genetic background of the hCD20-transgenic mice used, strongly influenced CD20-induced B-cell depletion, with stronger depletion in BALB/c than in C57BL/6 mice. In this context, it is important to note that serum complement activity differs significantly between mouse strains and varies with gender and age.4-6 In our own experience, BALB/c mice (males in particular) have relatively high levels of complement activity compared with C57BL/6 mice. In our view, this may explain the observed differences and identify complement activation as a significantly contributing factor to the in vivo mechanism of action of CD20 antibodies.
The contribution of complement activation to B-cell depletion by CD20 antibodies is strongly supported by several studies. Kennedy et al have shown that CD20+ cells are depleted concomitantly with the consumption of complement in patients during treatment with rituximab.7 Golay et al demonstrated that therapeutic activity against established B-cell tumors by rituximab required complement.8 Furthermore, Gong et al9 showed depletion of resident B-cells, such as those in the splenic marginal zone, to be critically dependent on complement. Notably, as above, the magnitude of complement-mediated B-cell depletion differed between mice of distinct genetic backgrounds. Circulatory B-cells (such as peripheral blood, lymph node, and splenic follicular B-cells), in contrast, can also be effectively cleared by Fc-receptor–mediated mechanisms in the absence of complement.9,10 The observations by Beers et al, which document depletion of circulating (CFSE-labeled) B-cells in mice deficient for C1q serves to confirm this notion.
We conclude that the data presented by Beers et al do not support conclusions that minimize a role for complement in B-cell depletion by CD20 antibodies. In contrast, the increased B-cell depletion in complement-sufficient BALB/c mice in fact supports a critical contribution. Therapeutic CD20 antibodies may engage multiple mechanisms to deplete B-cells, of which Fc-mediated mechanisms seem sufficient for clearance of circulatory cells, whereas depletion of resident normal and tumor B-cells appears to be critically dependent on complement.
Authorship
We thank Marleen Voorhorst and Hendrik ten Napel for performing CDC experiments.
Conflict-of-interest disclosure: The authors are employees of Genmab and own warrants and/or stock. Genmab is developing ofatumumab, a therapeutic CD20 antibody.
Correspondence: Dr Paul Parren, Genmab, Yalelaan 60, 3584 CM Utrecht, The Netherlands; e-mail: P.Parren@genmab.com.