Abstract
We studied the role of CD28 in T-cell biology and T cell–mediated pathology using a novel mouse anti–mouse CD28 antibody, E18, which recognizes an epitope close to the B7 binding site. In vitro, this antibody completely blocked binding of B7 molecules to CD28 expressed on mouse thymocytes but enhanced anti-CD3–induced proliferation of peripheral T cells. Injections of E18 monoclonal antibody into normal BALB/c mice in vivo, however, led to a reversible reduction in Treg cell frequencies among CD4+ cells, both in the thymus and in secondary lymphoid organs, suggesting that E18 acted as an inhibitor of CD28 signaling under these conditions. Antagonistic activity of E18 in vivo was further implied by suppressed responses of conventional CD4+ T cells to stimulation with the superantigen staphylococcal enterotoxin B and in a model of acute graft-versus-host disease. In contrast to healthy mice, intact monoclonal antibody E18, but not its nonstimulatory Fab fragment, increased the frequencies of Treg cells among CD4+ T cells in these pro-inflammatory settings allowing for efficacious protection from acute graft-versus-host disease. Thus, the agonistic signal generated by conventional, ie, nonsuperagonistic, anti-CD28 antibodies is important for their immunotherapeutic potential in vivo.
Introduction
Because of its important role in T-cell activation, the CD28-B7 axis lends itself as a target for immunotherapeutic interventions.1-4 To prevent CD28-B7 interactions, and thus priming of naive T cells, anti-CD28 antibodies,5 CTLA-4-immunoglobulin (Ig),6 or anti-CD80 and anti-CD86 antibodies have been used.7 Focusing on anti-CD28 antibodies, these antibodies fall into 2 classes with regard to their stimulatory activity on T cells: whereas so-called conventional anti-CD28 antibodies only act as costimulators in concert with T-cell receptor (TCR) complex triggering, superagonistic anti-CD28 monoclonal antibodies (mAbs) are capable of inducing full T-cell activation by themselves (ie, without TCR stimulation).8 Conventional anti-CD28 antibodies have successfully been applied in rodent models of human diseases, such as experimental autoimmune encephalomyelitis,9 experimental autoimmune neuritis,10 in vivo stimulation with staphylococcal enterotoxin B (SEB),11 solid organ transplantation,12 and acute graft-versus-host disease (aGVHD).13 In humans, the superagonistic anti-CD28 mAb TGN1412 was probably the first antihuman CD28 mAb to be tested clinically. Unexpectedly, a phase 1 trial and further clinical development of TGN1412 had to be stopped because of the massive release of inflammatory cytokines triggered by TGN1412 in humans.14 In animal models of human diseases, however, superagonistic anti-CD28 mAbs were unequivocally beneficial and therapeutically highly efficacious because of their potential to activate and expand regulatory T cells (Tregs).15-18 In contrast to the CD28 superagonists, the mode of action of conventional anti-CD28 mAbs in rodent models is controversial and may vary depending on the specific mAb and experimental setting used (reviewed by Hunig5 ). For example, the epitope binding site, in particular the ability to stimulate versus block endogenous ligands influences the outcome of in vivo application. Furthermore, the species origin of the mAb applied in mice (mostly hamster) will affect crystallizable fragment (Fc) receptor binding and half-life. To keep such uncertainties at a minimum, we have extensively characterized a novel mouse anti–mouse CD28 mAb, E18, which recognizes an epitope in the region of the interaction site with B7.19 E18, a mAb of the IgG2b isotype, is not only fully compatible with the murine immune system; it also is the first anti-CD28 mAb to completely inhibit CD28 ligation by B7 molecules. We took particular care in analyzing the impact of E18 treatment on the Treg cell compartment, given the importance of B7-CD28 signaling in Treg cell homeostasis in vivo.20-25
Methods
Animals
Normal BALB/c mice and CD90.1-congenic C57BL/6 (B6) mice were bred at the animal facility of the Institute for Virology and Immunobiology, University of Würzburg and used for experiments between 6 and 12 weeks of age. B6.OlaHsd bone marrow (BM) donors and BALB/c.OlaHsd hosts for aGVHD experiments were obtained from Harlan-Winkelmann (Borchen, Germany). All experiments were performed according to the Bavarian state regulations for animal experimentation and approved by the Regierung von Unterfranken as the responsible authority.
Fluorescence-activated cell sorter analysis
The following monoclonal antibodies were used: anti-CD25 fluorescein isothiocyanate (FITC), anti-CD90.1 phycoerythrin (PE) or biotin, anti-CD4 Alexa-647, anti-Vβ8 biotin, anti-B220 Alexa-647, anti-CD3 PE-Cy5 or PE-Cy7, purified anti-CD28 antibody 37.51 (all BD Biosciences PharMingen, San Diego, CA), and anti-Foxp3 PE or PE-Cy5 (eBioscience, San Diego, CA).
Stainings were performed with up to 106 cells in 50 μL of phosphate-buffered saline (PBS)/0.1% bovine serum albumin/0.02% NaN3. FcγRII/III receptors were blocked by incubation with saturating amounts of cell culture supernatant of the clone 2.4G2. Flurochrome-conjugated or biotinylated mAbs were added after blocking (15 minutes, 4°C). Bound biotinylated antibodies were detected by incubation with either PE-Cy5 or allophycocyanin conjugated streptavidin (BD Biosciences PharMingen). The cells were analyzed on a FACSCalibur flow cytometer using Cell Quest software (all BD Biosciences, San Jose, CA). Dot plots and histograms are shown as log10 fluorescence intensities on a 4-decade scale.
For intracellular staining of Foxp3, cells labeled with antibodies at the cell surface were fixed for 30 minutes at room temperature with fixation buffer (eBioscience) before permeabilization (permeabilization buffer; eBioscience). The cells were blocked with rat serum before staining with anti-Foxp3 mAb for 30 minutes at room temperature.
To study the inhibition of ligand binding to CD28 by E18 or E18 Fab, 106 thymocytes were resuspended in 50 μL of PBS/0.1% bovine serum albumin/0.02% NaN3 and incubated without or with different doses of intact E18 or its Fab fragment (1 hour, 4°C), followed by recombinant mouse CD80-human Ig fusion protein (5 μg/mL; R&D Systems, Minneapolis, MN). After washing, the binding of mouse CD80-human Ig to CD28 was revealed by anti–human Ig FITC. Staining of thymocytes without preincubation with mouse CD80-human Ig served as negative control.
E18 applications into healthy mice
E18 was either injected intraperitoneally in a single dose of 250 μg/mouse or in 25 repetitive doses of 100 μg/mouse with control animals receiving either the same dose of mAb MOPC-31C (mouse IgG1) or PBS injections as control treatment.
T-cell responses to SEB in vivo
To elicit a T-cell response to SEB in vivo, mice were injected intravenously with 50 μg of SEB. Anti-CD28 mAb E18 or control antibody PPV-06 (mouse IgG1) was injected intraperitoneally 2 hours before SEB stimulation. To directly follow the proliferative response of CD4+Vβ8+ T cells, 1 to 2 × 107 carboxyfluorescein succinimidyl ester diacetate (CFSE)–labeled (2.5 μM; MoBiTec, Göttingen, Germany) syngeneic lymph node cells were transferred intravenously into BALB/c recipients, followed by intraperitoneal mAb or Fab administration 22 hours later and SEB stimulation (intravenously) after 24 hours. Fab injections were repeated 24 hours and 48 hours after the first application. Three days after SEB injection, splenocytes from recipient animals were stained and CFSE-dye dilution among different T-cell subsets was determined. Fab fragments of E18 were prepared by ImmunoPure IgG1 Fab and F(ab′)2 Preparation Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. In brief, ficin was used for digestion and protein A columns were used for separation of the Fab fragments from undigested intact mAb or Fc fragment. The quality of the preparations was routinely checked by gel electrophoresis.
For depletion of CD4+CD25+ Treg cells, BALB/c mice received a single injection of 1 mg PC61 or, as a control, 250 μL of PBS intraperitoneally. Ten days later, CFSE-labeled CD25-depleted lymph node cells were transferred. followed by consecutive application of E18 or PPV-06 and SEB.
aGVHD experiments and tracking of allo–T-cell responses in vivo
BALB/c mice were conditioned for transplantation by total body irradiation with 8 Gy as a single dose. To prevent bacterial infections, animals were given neomycin (250 μg/mL; Sigma-Aldrich, St Louis, MO) and polymyxin B sulfate (3 U/mL; Sigma-Aldrich) in drinking water starting 3 days before irradiation until day 28 after transplantation. Approximately 24 hours after irradiation, the mice received 107 T cell–depleted (TCD) BM cells from B6 mice either alone (BM only) or together with 106 CD4+ or 8.5 × 105 CD4+CD25− peripheral T cells from CD90.1-congenic B6 mice. To obtain TCD BM cells, erythrocytes were lysed from total BM preparations, then Fc receptors were blocked with 20 μg/mL of normal mouse Ig (Sigma-Aldrich) before T cells were depleted using magnetic cell sorting anti-CD90.2 beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic cell sorting separation columns according to the manufacturer's instructions. T-cell depletion was approximately 95% on average. CD4+ T cells were purified from erythrocyte-lysed splenocytes with average purities of 90% by negative magnetic selection of cells expressing CD8α, CD11b, B220, CD49b, and/or Ter-119 (Miltenyi Biotec). CD25+ cells were depleted either by directly adding a biotinylated anti-CD25 antibody to the other lineage markers or by staining CD4+ cells with anti-CD25 FITC followed by anti-FITC beads (Miltenyi Biotec). In short-term experiments, CD90.1+ T cells were labeled with CFSE (2.5 μM) and 2.2 × 106 CD4+ or 2.0 × 106 CD4+ CD25− T cells were transferred. Independent of the observation period, 250 μg/mouse of mAb E18 (E18) or control mAb MOPC-31C (Ctrl) was injected intraperitoneally 16 hours after the TCD BM and T-cell transfer. Observers blinded to the treatment measured body weight and scored clinical appearance of the animals every other day as follows (scores in parentheses)26 : weight loss: less than 10% (0), more than 10% to less than 25% (1), more than 25% (2); posture: normal (0), hunching noted only at rest (1), severe hunching that impairs movement (2); activity: normal (0), mild to moderately decreased (1), stationary unless stimulated (2); fur texture: normal (0), mild to moderate ruffling (1), severe ruffling/ poor grooming (2); skin integrity: normal (0), scaling of paws/tail (1), obvious areas of denuded skin (2). At each observation time point, values were added to obtain one cumulative clinical scoring value per animal. Mice with less than 70% of the initial body weight for more than 2 days were killed. Alternatively, animals were killed independently of their body weight to prevent severe suffering as indicated by their overall clinical appearance.
Histology
Formalin-fixed small-bowel and large-bowel sections (5 μm) were stained with hematoxylin and eosin for histologic examination. An observer blinded to the prior treatment evaluated the following histopathologic changes (adapted from Hill et al27 and Cooke et al28 ): small bowel: lamina propria lymphocytic infiltrate, villous blunting, luminal sloughing of cellular debris, and outright crypt destruction; large bowel: lamina propria lymphocytic infiltrate, mucosal ulceration, and outright crypt destruction. The severity of pathologic changes was graded as follows (scores in parentheses): normal (0), focal and rare (0.5), focal and mild (1), diffuse and mild (2), diffuse and moderate (3), diffuse and severe (4). Scores were cumulated into a single value per animal.
Statistics
P values given either in the figures or in the text are the results of 2-tailed Student t tests assuming equal variance within groups. P less than .05 was considered statistically significant.
Results
Mouse anti–mouse CD28 mAb E18 blocks binding of B7 to CD28
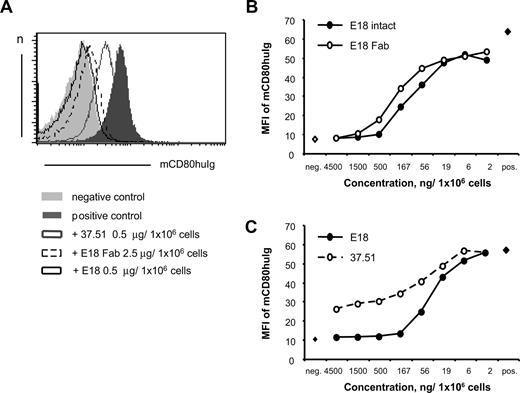
As E18 binds CD28 in the region of the B7 binding site,19 we analyzed the capacity of E18 to inhibit binding of recombinant mouse CD80-human Ig to mouse thymocytes, which are high in CD28 expression. As shown in Figure 1A, both E18 and its Fab fragment were capable of blocking mouse CD80-human Ig binding to CD28 expressed by thymocytes. In contrast to mAb 37.51, both E18 and E18 Fab fully inhibited ligand binding to CD28 in this assay when more than 500 or 1500 ng/106 cells were used, respectively (Figure 1B). Similar to what has been reported by Yu et al,13 who allowed CD28/mAb interaction to occur in solution, increasing the amount of mAb 37.51 to 4.5 μg/106 cells still rendered incomplete inhibition in our assay system (Figure 1C). Concomitant to ligand blockade, E18 generated a costimulatory signal after binding to CD28 as evidenced by enhanced T-cell proliferation in conjunction with anti-CD3 stimulation in vitro (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Agonistic anti-CD28 mAb E18 is thus a potent inhibitor of CD28/B7 interactions and the first anti-CD28 mAb described, which completely blocks B7 binding to CD28.
Biochemical properties of mouse antimouse CD28 mAb E18. (A) Anti-CD28 mAb E18 blocks binding of B7 to CD28. Mouse thymocytes were incubated with E18, E18 Fab, or mAb 37.51 as reference before binding of mouse CD80-human Ig (mCD80huIg) was assessed. (B) Titration of E18, its Fab fragment, and 37.51 (C) in assays analyzing ligand blocking activity as in panel A.
Biochemical properties of mouse antimouse CD28 mAb E18. (A) Anti-CD28 mAb E18 blocks binding of B7 to CD28. Mouse thymocytes were incubated with E18, E18 Fab, or mAb 37.51 as reference before binding of mouse CD80-human Ig (mCD80huIg) was assessed. (B) Titration of E18, its Fab fragment, and 37.51 (C) in assays analyzing ligand blocking activity as in panel A.
Application of E18 into healthy mice in vivo inhibits thymic differentiation of Treg cells and induces a reversible reduction in the frequency of Treg cells among peripheral CD4+ T cells
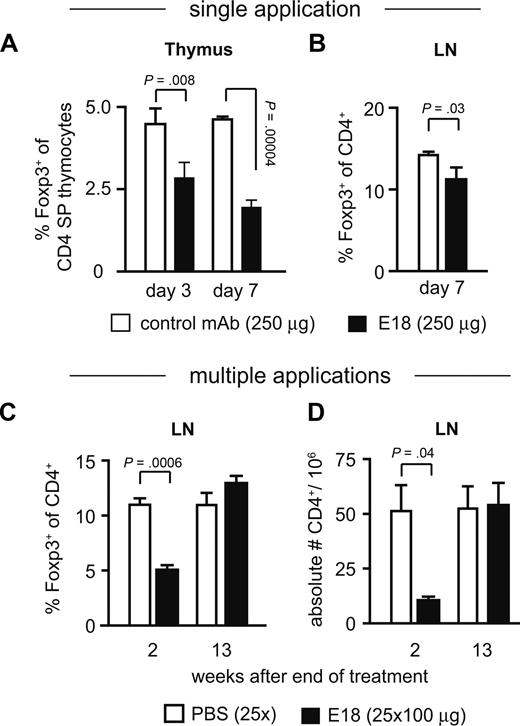
As among all T cells, Treg cells are most highly dependent on CD28-mediated signals,20,21,23 we followed Treg cell generation in the thymus and peripheral maintenance as a first readout for the biologic activity of E18 in vivo. The frequency of Foxp3+ cells among CD4 single-positive thymocytes was reduced by 50% 7 days after a single application of E18 into healthy B6 mice (Figure 2A). Among CD4+ cells of peripheral lymph nodes, Treg cell frequencies were still at 90% compared with control mAb-treated controls 7 days after E18 injection (Figure 2B). Addition of E18 to newborn thymus organ cultures in vitro also inhibited the generation of Treg cells (G.B., T.H., unpublished data, April 2006). With E18 being a mouse anti–mouse CD28 mAb, we could perform long-term treatment of mice, injecting the antibody 25 times at weekly intervals. This continued application of E18, which is of the IgG2b isotype, was well tolerated by the treated animals. Two weeks after the final injection, ie, when E18 still occupied CD28 at saturating levels (G.B., T.H., unpublished data, April 2006), Treg cell frequencies among CD4+ cells in peripheral lymph nodes were reduced only to approximately 50% of that of PBS-treated controls (Figure 2C), and 13 weeks after cessation of E18 treatment the Treg cell pool was fully replenished (Figure 2C). Similar to anti-CD25 mAb-treated mice,29 E18-injected mice did not develop any detectable clinical or serologic signs of “spontaneous” autoimmunity (G.B., T.K., and T.H., unpublished data, April 2006), despite low Treg cell frequencies for several weeks.
In vivo application of E18 into healthy BALB/c mice. (A) Relative frequencies of Foxp3+ cells among CD4 single-positive thymocytes and CD4+ lymph node cells (B) were determined 7 days after intraperitoneal application of 250 μg of E18 or control mAb into BALB/c mice. Means plus or minus SD of 3 mice per group are depicted. (C) BALB/c mice received one injection per week of either PBS or 100 μg of E18 for a total of 25 weeks. Two and (D) 13 weeks after the last injection, lymph node cells were analyzed for the frequencies of Foxp3+ cells among CD4+ cells and the absolute numbers of CD4+ T cells. Means plus or minus SD of 3 to 4 mice per group and time point are depicted.
In vivo application of E18 into healthy BALB/c mice. (A) Relative frequencies of Foxp3+ cells among CD4 single-positive thymocytes and CD4+ lymph node cells (B) were determined 7 days after intraperitoneal application of 250 μg of E18 or control mAb into BALB/c mice. Means plus or minus SD of 3 mice per group are depicted. (C) BALB/c mice received one injection per week of either PBS or 100 μg of E18 for a total of 25 weeks. Two and (D) 13 weeks after the last injection, lymph node cells were analyzed for the frequencies of Foxp3+ cells among CD4+ cells and the absolute numbers of CD4+ T cells. Means plus or minus SD of 3 to 4 mice per group and time point are depicted.
Whereas Treg cells were more profoundly affected than other CD4+ T-cell subsets, the effect was not Treg cell-specific as indicated by the overall loss of CD4+ cells from lymph nodes (Figure 2D). CD4+ T-cell numbers in E18-treated animals ranged at 20% of PBS controls, however, again rebounding to normal values when E18 applications had been stopped. Absolute numbers of CD8+ T cells were not reduced after E18 treatment (G.B., T.H., unpublished data, April 2006). Whereas the precise reasons for the reduction in total CD4+ cell numbers remain to be resolved, observations in B7-transgenic and -deficient mice30 and lack of evidence for direct depletion of T cells by E18 (X.D., N.B., T.K., and T.H., unpublished data, June 2007) suggest that spatial and/or temporal modulation of CD28 signaling by E18 accounts for this phenomenon. This notion is also consistent with the observation that the injection of mAb E18 interfered with the generation of Treg cells in the thymus and in thymic organ cultures. Among peripheral CD4+ T cells, E18 treatment induced a decline in overall numbers, whereas Treg cell frequencies were reversibly and altogether less reduced than in the thymus. This is in marked contrast to the aforementioned superagonistic mAbs, which induce a general increase in CD4+ T cells dominated by an expansion of Treg cells.5
After SEB stimulation, mAb E18 preferentially blocks the CD4+ T-cell response of Foxp3− cells over that of Foxp3+ cells
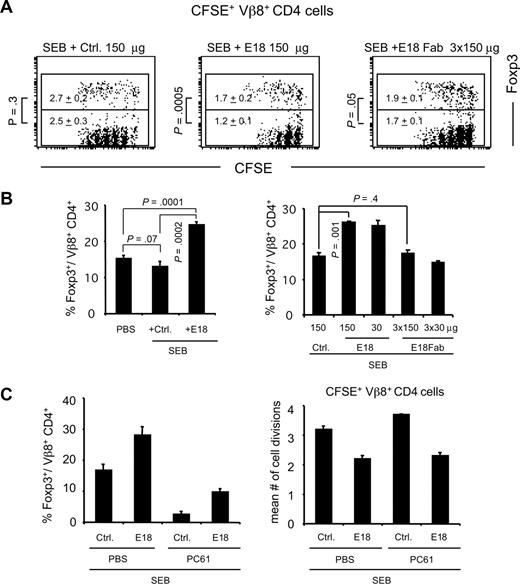
Because in normal mice E18 treatment reduced the frequency of Treg cells among CD4+ cells (Figure 2), we asked whether Foxp3+ and Foxp3− CD4+ cells were also differentially affected after E18 application in the context of an immune response in vivo. Stimulation of mice with SEB and analysis of CFSE dye dilution profiles among SEB-responsive Vβ8+ donor T cells revealed that E18 application diminished the mean number of cell divisions among both Foxp3+ and Foxp3− CD4+ cells (Figure 3A). Foxp3+ cells were, however, less affected than Foxp3− cells, with the mean number of cell divisions of Foxp3+Vβ8+CD4+ cells still at 63% of controls (Figure 3A top figures: 1.7 [E18], 2.7 [Ctrl]) versus 48% for Foxp3− cells (Figure 3A bottom figures: 1.2 (E18), 2.5 [Ctrl]). This bias in proliferative activity toward Foxp3+ cells after E18 application was reflected by an increase in the frequencies of Foxp3+ cells among transferred (X.D., N.B., T.K., and T.H., unpublished data, November 2006) and “endogenous” Vβ8+CD4+ cells 3 days after SEB and E18 application compared with SEB plus control mAb (Figure 3B). Treatment of mice with E18 Fab led to an almost parallel reduction in the mean number of cell divisions among Foxp3+ and Foxp3− cells to approximately 70% and 68%, respectively, of controls (Figure 3A) and had no significant impact on the frequencies of Foxp3+ cells among Vβ8+CD4+ cells (Figure 3B right bar graph).
Impact of E18 on the CD4+ TCR to SEB in vivo. (A) Analysis of CFSE dye dilution among adoptively transferred CD4+Vβ8+Foxp3+ and Foxp3− cells. The figures represent the mean numbers of cell divisions of Foxp3+ cells (top values within each dot plot) and Foxp3− cells (bottom values within each dot plot). (B) Average frequencies of Foxp3+ cells among Vβ8+CD4+ cells on day 3 after the indicated treatments. (C) Mice were depleted of Treg cells by anti-CD25 mAb treatment, and Treg cell frequencies among host Vβ8+ CD4 cells were analyzed 3 days after SEB injection (left); the mean number of cell divisions among transferred CD25-depleted CFSE+ cells was calculated (right). Bars represent means plus or minus SD of 2 to 5 mice per group.
Impact of E18 on the CD4+ TCR to SEB in vivo. (A) Analysis of CFSE dye dilution among adoptively transferred CD4+Vβ8+Foxp3+ and Foxp3− cells. The figures represent the mean numbers of cell divisions of Foxp3+ cells (top values within each dot plot) and Foxp3− cells (bottom values within each dot plot). (B) Average frequencies of Foxp3+ cells among Vβ8+CD4+ cells on day 3 after the indicated treatments. (C) Mice were depleted of Treg cells by anti-CD25 mAb treatment, and Treg cell frequencies among host Vβ8+ CD4 cells were analyzed 3 days after SEB injection (left); the mean number of cell divisions among transferred CD25-depleted CFSE+ cells was calculated (right). Bars represent means plus or minus SD of 2 to 5 mice per group.
Depletion of CD25+ cells before transfer of CFSE-labeled lymph node indicator cells revealed that E18 administration predominantly induced a relative increase in the frequency of preexisting Foxp3+ cells with little or no de novo expression of Foxp3 by conventional CD4+ T cell (Tconv) cells (X.D., N.B., T.K., and T.H., unpublished data, November 2007).
The increase in Treg cell frequencies after E18 and SEB administration suggested that Treg cells might actively contribute to the reduction in Vβ8+ T-cell proliferation after E18 application. To address this question, we depleted BALB/c mice of Foxp3+ cells by injection of the anti-CD25 mAb PC6131 before E18 or control mAb and SEB application (Figure 3C left bar graph). Whereas the Treg cell/Tconv cell ratio among Vβ8+CD4 cells was reduced from 1:5 (nondepleted) to 1:38 in anti-CD25 mAb- and control mAb-treated animals, E18 application led to a disproportionate increase in the frequencies of these remaining Treg cells amounting to a Treg cell/Tconv cell ratio of 1:9 in Treg cell–depleted, E18-treated mice versus 1:3 in nondepleted mice (Figure 3C, left bar graph). When we analyzed the mean number of cell divisions of CFSE-labeled, CD25-depleted Vβ8+CD4+ indicator cells, which we had transferred into the same animals, anti-CD25 mAb application in vivo led to an increase from 3.2 to 3.7 (Figure 3C right panel). Irrespective of anti-CD25 mAb treatment, E18 application reduced the proliferation of the indicator T cells to 2.2 and 2.3 divisions on average, respectively (Figure 3C right panel).
During SEB responses, mAb E18 thus provides preexisting Treg cells with a relative proliferative advantage over Tconv cells, leading to an increase in their frequencies among CD4+ cells, which was even enhanced after anti-CD25 mAb-mediated partial depletion of Treg cells. This, in turn, might have allowed for full inhibition of the response of Vβ8+CD4+ Tconv cells to SEB by E18 in mice with reduced initial Treg cell frequencies.
Efficient protection from aGVHD by E18 in the presence of donor Treg cells
These findings are in accordance with the observation that after activation very few Treg cells are sufficient to confer protection from immunopathology in vivo.18 To assess the therapeutic efficacy of E18 in a situation with very low total numbers of Treg cells per animal and to evaluate its capacity to protect from immunopathologic changes in vivo, we used a model of aGVHD. We treated lethally irradiated BALB/c recipients of B6 BM and 106 B6.CD90.1+CD4+ (21.0% Foxp3+ cells) or 8 × 105 CD4+CD25− T cells (5.9% Foxp3+ cells) with 250 μg/animal of either E18 or control mAb one day after BM and T-cell transplantation (Figure 4A). Whereas control mAb-treated animals rapidly died of aGVHD (mean survival time for recipients of CD4+ cells, 13 days), the majority of E18-treated mice survived until the end of the observation period (mean survival time (CD4+), 58 days; P = .02). Mice inoculated with CD4+CD25− donor T cells showed slightly enhanced survival after E18 application compared with control mAb-treated mice (mean survival time, 29 days vs 7 days; P = .2). Apart from better survival, injection of E18 also reduced signs of aGVHD as determined by clinical scoring (Figure 4B). On day 8, that is, when the clinical scores of control mAb-treated animals had reached plateau levels with a mean score of 4.2, animals receiving CD4+ T cells and E18 treatment had developed clinical disease scored only 2.0 on average (P = .03). Injection of E18 into animals transplanted with CD4+CD25− T cells (score, 3.6; control mAb-injected controls: score, 4.2, P = .3) was less efficacious than in recipients of whole CD4+ T cells.
Treatment of aGVHD with E18 mAb. (A) Lethally irradiated BALB/c mice were reconstituted with 107 B6 TCD BM cells either alone or together with 106 CD4+ (n = 3 experiments) or 8 × 105 CD4+CD25− peripheral T cells (n = 2 experiments) from CD90.1-congenic B6 mice. E18 mAb was administered 1 day after the cell transfer. The percentages of animals surviving over time are depicted as Kaplan-Meier graph. (B) Mean clinical scores of recipient animals (n = 5). (C) Representative hematoxylin and eosin stainings of small-bowel and large-bowel sections obtained from mice 6 days after transplantation (original magnification ×200): microscope, LEICA DMIRE2 (Leica Microsystems, Wetzlar, Germany); lens: HC PLAN S 10×/0.40 PH1; mounting medium: Entellan (Merck, Darmstadt, Germany); camera, LEICA DFC300 FX; acquisition software, LEICA IM50 Image Manager. Adobe Photoshop CS3 (Adobe Systems, San Jose, CA) was used to adjust for brightness and contrast and to convert to grayscale. (D) Histopathologic changes were scored on small-bowel and large-bowel sections as detailed in “Methods.” Cumulative scores are depicted for individual animals (○). Horizontal bars represent the medians per group. Data from 3 experiments were pooled. (E) The percentages of Foxp3+ cells among donor-derived CD4+ cells isolated from spleen, MLN, or liver were determined on day 6 after transplantation. ○ represent individual animals.  represent the median values of each group with data from 4 experiments being pooled. Ctrl indicates control mAb treatment.
represent the median values of each group with data from 4 experiments being pooled. Ctrl indicates control mAb treatment.
Treatment of aGVHD with E18 mAb. (A) Lethally irradiated BALB/c mice were reconstituted with 107 B6 TCD BM cells either alone or together with 106 CD4+ (n = 3 experiments) or 8 × 105 CD4+CD25− peripheral T cells (n = 2 experiments) from CD90.1-congenic B6 mice. E18 mAb was administered 1 day after the cell transfer. The percentages of animals surviving over time are depicted as Kaplan-Meier graph. (B) Mean clinical scores of recipient animals (n = 5). (C) Representative hematoxylin and eosin stainings of small-bowel and large-bowel sections obtained from mice 6 days after transplantation (original magnification ×200): microscope, LEICA DMIRE2 (Leica Microsystems, Wetzlar, Germany); lens: HC PLAN S 10×/0.40 PH1; mounting medium: Entellan (Merck, Darmstadt, Germany); camera, LEICA DFC300 FX; acquisition software, LEICA IM50 Image Manager. Adobe Photoshop CS3 (Adobe Systems, San Jose, CA) was used to adjust for brightness and contrast and to convert to grayscale. (D) Histopathologic changes were scored on small-bowel and large-bowel sections as detailed in “Methods.” Cumulative scores are depicted for individual animals (○). Horizontal bars represent the medians per group. Data from 3 experiments were pooled. (E) The percentages of Foxp3+ cells among donor-derived CD4+ cells isolated from spleen, MLN, or liver were determined on day 6 after transplantation. ○ represent individual animals.  represent the median values of each group with data from 4 experiments being pooled. Ctrl indicates control mAb treatment.
represent the median values of each group with data from 4 experiments being pooled. Ctrl indicates control mAb treatment.
To assess aGVHD activity in the different clinical scenarios by histopathology, we scored hematoxylin and eosin-stained paraffin sections of the small and large bowels from mice killed 6 days after T-cell transplantation (Figure 4C). Small-bowel and, even more so, large-bowel sections of control mAb-treated mice were characterized by profound lymphocytic infiltrates into the lamina propria accompanied by marked destruction of the gut architecture, which was largely unaltered in mice receiving E18 (Figure 4C). For summary, data from both organs were integrated into a cumulative score (see “Methods” and Figure 4D): Control mAb application in conjunction with a total CD4+ T-cell inoculum resulted in a median score of 22, whereas in E18-treated animals pathologic changes were rated with a median of 3.5 (Figure 4D). In mice receiving CD4+CD25− T cells, control mAb-treated animals developed gut pathology with a median value of 18.3 vs 7.5 after injection of E18 (Figure 4D).
Because protection from aGVHD by E18 was most efficacious when total CD4+ cells had been transplanted and the ratio of Treg cells to conventional T cells is a sensitive indicator for immune modulation by Treg cells in vivo, we analyzed the frequencies of Foxp3+ cells among the progeny of donor CD90.1+CD4+ T cells 6 days after transplantation. MAb E18 elicited an increase of almost 3-fold in the frequencies of Foxp3+ cells among nondepleted and CD25-depleted donor T cells isolated from mesenteric lymph nodes (MLNs), compared with control mAb-treated animals (Figure 4E). Treg cell frequencies in the spleen and among donor T cells retrieved from the liver, a major target organ of aGVHD, were, however, not increased by E18 treatment (Figure 4E). Together, our data indicate that protection from aGVHD by E18 was most efficacious in the presence of donor-derived Treg cells, which were increased in frequency among donor T cells recovered from MLNs, but not among donor T cells recovered from spleen or liver.
Impaired infiltration of Foxp3− pathogenic T cells into aGVHD target organs after E18 treatment protects from immunopathology
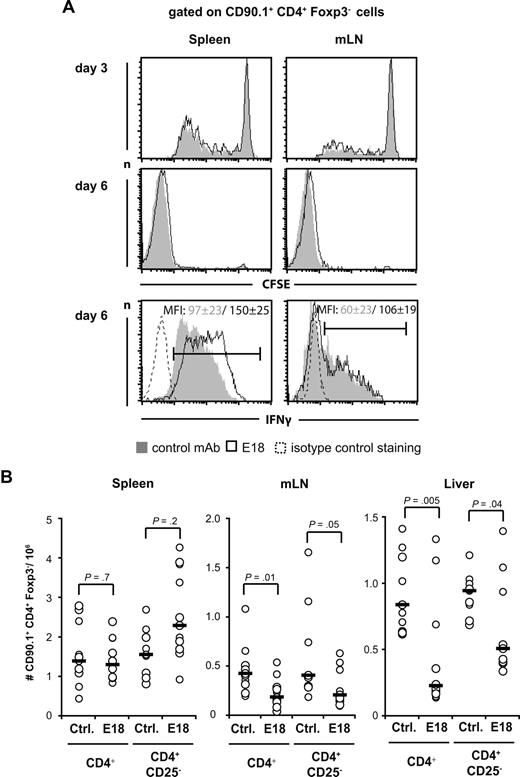
One mechanism by which E18 could mediate protection from aGVHD is to interfere with the initial clonal expansion and effector cell differentiation of allogeneic Foxp3− Tconv cells in the spleen or the gut-draining MLNs, which constitute the 2 hot spots early during aGVHD.32 We labeled CD4+CD90.1+ cells with CFSE before transfer into allogeneic hosts and followed CFSE dye dilution among Foxp3− cells isolated from spleen and MLN 3 and 6 days after transplantation (Figure 5A). Interferon-γ (IFN-γ) production by transplanted Foxp3− T cells was determined on day 6 (Figure 5A). Neither CFSE dye dilution among Foxp3− cells nor the capacity of Foxp3− donor T cells to produce IFN-γ was decreased by E18 application (Figure 5A). Whereas CFSE dilution profiles were almost congruent, IFN-γ expression by Foxp3− donor T cells restimulated with phorbol myristate acetate/ionomycin was even increased by a factor of approximately 1.5 after E18 compared with control mAb treatment. Transfer of CD4+CD25− T cells rendered similar results to those in Figure 5A (N.B., T.K., October 2007). Equal degrees of CFSE dye dilution were mirrored by the accumulation of similar numbers of donor-derived Foxp3− T cells in the spleens of E18- and control mAb-treated animals (Figure 5B, left diagram). In MLNs, however, E18 treatment reduced the number of CD90.1+CD4+Foxp3− cells retrieved by approximately 2-fold, irrespective of whether CD4+CD25− or total CD4+ cells had been transferred (Figure 5B middle diagram).
Tracking of Foxp3- alloreactive T-cell expansion and differentiation after transplantation and E18 application. (A) CFSE-dye dilution among and IFN-γ production by donor CD4+ T cells recovered from spleen (left) or MLN (right) 3 and 6 days after transplantation into lethally irradiated BALB/c mice and 5 days after control mAb (Ctrl, gray shadow) or E18 (black line) application. The figures indicate mean fluorescence intensities (MFI) plus or minus SD of cells selected by the marker. (B) Absolute numbers of donor CD4+Foxp3− T cells in spleen (left), MLN (middle), and liver (right) 6 days after transplantation were calculated by multiplying absolute cell number per organ with the percentage of cells as determined by fluorescence-activated cell sorter analysis. Circles represent individual animals. Horizontal bars represent median values with data from 4 experiments being pooled.
Tracking of Foxp3- alloreactive T-cell expansion and differentiation after transplantation and E18 application. (A) CFSE-dye dilution among and IFN-γ production by donor CD4+ T cells recovered from spleen (left) or MLN (right) 3 and 6 days after transplantation into lethally irradiated BALB/c mice and 5 days after control mAb (Ctrl, gray shadow) or E18 (black line) application. The figures indicate mean fluorescence intensities (MFI) plus or minus SD of cells selected by the marker. (B) Absolute numbers of donor CD4+Foxp3− T cells in spleen (left), MLN (middle), and liver (right) 6 days after transplantation were calculated by multiplying absolute cell number per organ with the percentage of cells as determined by fluorescence-activated cell sorter analysis. Circles represent individual animals. Horizontal bars represent median values with data from 4 experiments being pooled.
After initial activation in secondary lymphoid organs, the alloreactive T cells migrate into peripheral tissues, of which liver, gut, and skin are the most severely affected. We quantitated donor CD90.1+CD4+Foxp3− T-cell infiltration into the livers of mice undergoing aGVHD (Figure 5B right panel). In mice transplanted with total CD4+ T cells, E18 treatment reduced the absolute number of intrahepatic donor Foxp3− T cells isolated on day 6 after transplantation by a factor of 3 compared with control mAb-treated controls (Figure 5B right panel). When CD4+CD25− T cells had been transplanted, donor T-cell infiltration was reduced approximately 2-fold (Figure 5B right panel).
Taking these findings together, protection from aGVHD by E18 application was not associated with reduced proliferation of Foxp3− donor T cells, but with a lower burden of pro-inflammatory T cells as evidenced by reduced accumulation and less infiltration of Foxp3− donor T cells in MLN and the liver, respectively.
Discussion
In this study we have addressed the link between the ligand-blocking properties of mAb E18 in vitro and its impact on T-cell responses in vivo to further understand the mechanisms underlying immunotherapy with conventional anti-CD28 mAbs.
A key feature of mAb E18 is its capacity to fully inhibit the interaction of membrane-bound CD28 with its natural ligand B7. Although the epitope recognized by the widely used anti-CD28 antibody 37.51 was also mapped to the region of the B7-binding site on the CD28 molecule,33 37.51 was only capable of partially blocking CD28/B7 interactions (Figure 1). This finding is in agreement with an earlier study13 describing almost no blockade of mouse CD28-human Ig binding to lipopolysaccharide blasts by 37.51. Inhibition of ligand binding by the also frequently used conventional anti-CD28 mAb PV-19,34,35 has not been directly addressed. Therefore, E18 is the first anti–mouse CD28 antibody described to fully block ligand binding to CD28.
In accordance with ligand blockade by E18, in vitro thymic generation of Treg cells was markedly inhibited in vivo (Figure 2A), whereas peripheral Treg cells were hardly affected (Figure 2B). Seemingly contradictory to this reduction in thymic Treg cell output after E18 application into healthy mice, however, treatment with E18, but not the Fab fragment, increased Treg cell frequencies among CD4+ cells in the SEB system and during aGVHD (Figures 3, 4). Thus, in the presence of allo- or superantigen and other pro-inflammatory factors, E18's agonistic activity (Figures S1,S2) most probably enhanced interleukin-2 production by conventional T cells, allowing for this relative increase in Treg cell frequencies. Conversely, stimulation of CD28 on Treg cells by E18 without provision of additional interleukin-2 by conventional T cells might have exerted a direct inhibitory effect on Treg cells of healthy mice (Figure 2).
Whereas the relative increase in Treg cell frequencies after E18 application in vivo (Figure 3B) suggested that Treg cells actively contributed to the overall inhibition of the T-cell response to SEB (Figure 3A), partial depletion of Treg cells in vivo did not reduce the capacity of E18 to inhibit the T-cell response to SEB (Figure 3C). Therefore, E18 either exerted a strong direct inhibitory effect on Vβ8+ Tconv cells or the markedly increased Treg cell/Tconv cell ratio in TCD animals, and the absolute number of Treg cells per animal in response to E18 application was sufficient to allow for full Treg cell-mediated suppression (Figure 3C).
In the aGVHD model, elimination of the majority of Foxp3+ Treg cells before transplantation into the allogeneic host clearly reduced the protective effect of E18 on clinical disease (Figure 4). In contrast to the subtotal depletion of Treg cells in the nonlymphopenic SEB system, we think that during aGVHD the very low total number of Treg cells after transplantation of CD4+CD25− allogeneic T cells or that Treg cell–independent inhibitory mechanisms allowed for only a partial reduction of target organ infiltration by pathologic T cells and histopathologic changes to the gut mucosa after E18 treatment, which was below the threshold for full clinical protection (Figures 4,5).
Analysis of Treg cell frequencies in spleen and MLNs, the main priming sites during aGVHD induction,32 identified the MLNs as a hot spot for immunoregulation after E18 application as there, but not in the spleen; Treg cell frequencies were disproportionately increased among the progeny of both nondepleted and CD25-depleted CD4+ donor T cells (Figure 4). Higher Treg cell frequencies in MLNs after E18 treatment were accompanied by a reduction in the absolute number of pathogenic Foxp3− CD4+ T cells (Figure 5B). This could either be a consequence of reduced T-cell proliferation or because of increased death of postmitotic cells. Whereas T-cell proliferation and effector cell differentiation analyzed by CFSE dye dilution and IFN-γ production were either not reduced or even enhanced after E18 application (Figure 5A), we observed an increased proportion of annexin V+ cells among postmitotic (CFSElow) Foxp3− donor T cells in MLN after E18 therapy (Figure S3). A recent study revealed the link between apoptosis induction in conventional T cells and protection from aGVHD by Treg cells by identifying the cytokine withdrawal from Tconv cells by Treg cells observed in other systems36,37 as the main mechanism of Treg cell-mediated suppression in aGVHD.38 Yu et al,13 too, observed induction of apoptosis in allogeneic T cells after treatment of aGVHD with the anti-CD28 mAb 37.51. In light of our results and the proposed mode of action of Treg cells in aGVHD, it may well be that immunotherapy of aGVHD with 37.51 might also work, at least in part, through Treg cells with secondary apoptosis of conventional T cells.
In addition to the secondary induction of apoptosis, direct depletion of pathogenic T cells by E18 most probably did not substantially contribute to E18's protective effect as donor T-cell numbers were even slightly increased in the spleens after transplantation of CD4+CD25− T cells and E18 application (Figure 5B). E18 therapy might, however, have interfered with the homing of pro-inflammatory T cells into aGVHD target organs, thus protecting from aGVHD pathology.39-41
Taken together, blockade of CD28/B7 interactions by mAb E18 in vitro and interference with Treg cell generation in the thymus in vivo suggested that E18 would act primarily as an inhibitor of costimulation in vivo. However, the selective increase in Treg cell frequencies by E18, but not its Fab fragment, after SEB stimulation in vivo together with the enhanced suppression of aGVHD by E18 in the presence of physiologic frequencies of donor-Treg cells indicated that the agonistic signal generated by E18 mAb was important for its inhibitory properties in vivo. This result is reminiscent of recent findings for ligand-blocking anti-CD152 antibodies, which also exert their biologic activity, not through blockade of CD152 signaling, but through their capacity to stimulate CD152.42
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nadine Pfeifer and Sandra Werner for conducting experiments and providing excellent technical assistance and Christian Linden for helping with BM preparations.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Hu295/8) and the Wilhelm Sander-Stiftung (2005.133.1).
Authorship
Contribution: N.B. designed and performed research, performed statistical analysis, and wrote the manuscript; X.D. performed research and statistical analysis; G.B. performed research; K.M.D. provided vital new reagents and wrote the manuscript; and T.K. and T.H. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niklas Beyersdorf, Universität Würzburg, Institut für Virologie und Immunbiologie, Versbacherstr. 7, D-97078 Würzburg, Germany; e-mail: niklas.beyersdorf@vim.uni-wuerzburg.de.