Abstract
Stroke is predicted by abnormally high cerebral velocities by transcranial doppler (TCD). This study aimed at defining predictive factors for abnormally high velocities (≥ 2 m/sec) based on the Créteil pediatric sickle cell anemia (SCA) cohort composed of 373 stroke-free SCA children. α genes and β-globin haplotypes were determined. Biologic parameters were obtained at baseline. α-thalassemia was present in 155 of 325 and G6PD deficiency in 36 of 325 evaluated patients. TCD was abnormal in 62 of 373 patients. Multivariate logistic regression analysis showed that G6PD deficiency (odds ratio [OR] = 3.36, 95% confidence interval [CI] 1.10-10.33; P = .034), absence of alpha-thalassemia (OR = 6.45, 95% CI 2.21-18.87; P = .001), hemoglobin (OR per g/dL = 0.63, 95% CI 0.41-0.97; P = .038), and lactate dehydrogenase (LDH) levels (OR per IU/L = 1.001, 95% CI 1.000-1.002; P = .047) were independent risk factors for abnormally high velocities. This study confirms the protective effect of alpha-thalassemia and shows for the first time that G6PD deficiency and hemolysis independently increase the risk of cerebral vasculopathy.
Introduction
Predicting the severity of sickle cell anemia (SCA) is important for providing better informed genetic counseling and for better targeting of intensive therapies. Stroke is the most severe complication affecting children with SCA and occurs in 11% of SCA patients by the age of 18 years.1,2 The risk for stroke can be inferred from abnormally high cerebral velocities assessed by transcranial Doppler (TCD)3,4 and can be diminished by transfusion programs5 that cannot safely be stopped, even in patients with normalized velocities and normal magnetic resonance angiography (MRA).6,7 Therefore, while abnormally high velocities are useful to identify patients with severe profile, it would be important to define the risk factors associated with high velocities.
Methods
This study was based on the Créteil Pediatric Stroke-Free SCA (SS) cohort. It was approved by the Créteil Institutional Review Board and performed in accordance with the Declaration of Helsinki. Since TCD was introduced in Créteil in May 1992,8 patients have been screened by TCD annually from the age of 12-18 months. Details of the TCD imaging process are provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Time-averaged mean of maximum velocities (TAMMX) higher than 2 m/sec (middle, internal carotid, or anterior cerebral arteries) were considered as abnormal and resulted in immediate initiation of transfusion programs (see Document S1). α genes and β-globin haplotypes were determined. Baseline biologic parameters, ie, G6PD activity (reduction of NADP to NADPH measured on an ultraviolet spectrophotometer); white blood cells (WBC), neutrophils, reticulocytes, and platelet counts (109/L); hemoglobin (g/dL), hematocrit, fetal hemoglobin (HbF), and lactate dehydrogenase (LDH) levels (IU/L), and mean corpuscular volume (MCV; flt) were obtained after 12 months of age and, a minimum of 3 months away from a transfusion, one month from a painful episode and before intensive therapy (hydroxyurea, transfusion program, or stem cell transplantation). Variables in different groups of patients were compared using Wilcoxon test. Association between abnormal TCD and other variables was assessed using logistic regression. Multivariate analysis used stepwise regression procedure and the standardized beta option. Statistical analysis was performed with SAS 8.2 software (SAS, Cary, NC).
Results and discussion
Stroke-free SS children (n = 373; 180 females, 193 males) were examined annually by TCD.7-10 The results of 1352 annual TCD assessments in SS patients were recorded in the Center database. The mean number of measurements per patients was 2.35 (SD 2.26), and the range was from 1 to 13. Mean TCD follow-up was 22.6 months (SD 39.4), ranging from 0 to 201 months.
α genes study, available in 325 patients, demonstrated presence of α-thalassemia in 155 patients (47.7%), with deletion of either one (125, 38.5%) or 2 genes (30, 9.2%). β-globin haplotypes, studied in 293 patients, were homozygotes Car (40%), Ben (24%), Sen (9%), or “other” (27%). G6PD deficiency was present in 36 (28 males, 8 females) of 325 evaluated patients (11.1%). Velocities more than or equal to 2 m/sec were found in 62 of 373 stroke-free patients (16.6%). In the patients followed since birth (n = 187), abnormal high velocities occurred at the median age of 3.1 years, ranging from 1.5 to 8.3 years.
Results of the univariable predictive analyses are reported in Tables 1 and 2. Prevalence of abnormally high velocities was significantly decreased by the presence of α-thalassemia, high Hb and Hct; however, it was significantly increased by G6PD deficiency, high WBC counts, high reticulocyte counts, high MCV, and high LDH levels. There was no evidence of any effects of sex (male vs female), β-globin haplotypes, polymorphonuclear neutrophils (PMN), or HbF level on the risk for high velocities.
Predictive factors for abnormally high velocities (≥ 2m/sec) by univariable models
| Variable . | Stroke-free SS patients, n = 373 . | Abnormal TCD n = 62 . | Normal TCD n = 311 . | OR (95% CI) . | P . | ||
|---|---|---|---|---|---|---|---|
| Events . | n . | Events . | n . | ||||
| Male gender | 193 | 32 | 62 | 161 | 311 | 1.04 (0.61-1.77) | .89 |
| β-globin haplotypes | 293 | 42 | 251 | ||||
| Car/Car | 118 | 18 | 100 | 0.71 (0.38-1.33) | .28 | ||
| Ben/Ben | 71 | 10 | 61 | 0.60 (0.28-1.30) | .19 | ||
| Sen/Sen | 27 | 2 | 25 | 0.61 (0.20-1.87) | .39 | ||
| Others | 77 | 12 | 65 | ||||
| α-thalassemia | 155/325* | 12 | 50 | 143 | 275 | 0.29 (0.15-0.58) | < .001 |
| G6PD deficiency | 36/325* | 11 | 55 | 25 | 270 | 2.45 (1.12-5.35) | .024 |
| Variable . | Stroke-free SS patients, n = 373 . | Abnormal TCD n = 62 . | Normal TCD n = 311 . | OR (95% CI) . | P . | ||
|---|---|---|---|---|---|---|---|
| Events . | n . | Events . | n . | ||||
| Male gender | 193 | 32 | 62 | 161 | 311 | 1.04 (0.61-1.77) | .89 |
| β-globin haplotypes | 293 | 42 | 251 | ||||
| Car/Car | 118 | 18 | 100 | 0.71 (0.38-1.33) | .28 | ||
| Ben/Ben | 71 | 10 | 61 | 0.60 (0.28-1.30) | .19 | ||
| Sen/Sen | 27 | 2 | 25 | 0.61 (0.20-1.87) | .39 | ||
| Others | 77 | 12 | 65 | ||||
| α-thalassemia | 155/325* | 12 | 50 | 143 | 275 | 0.29 (0.15-0.58) | < .001 |
| G6PD deficiency | 36/325* | 11 | 55 | 25 | 270 | 2.45 (1.12-5.35) | .024 |
α gene study and G6PD were available in 325 of 373 patients.
Parameters at baseline in patients with abnormal or normal TCD and predictive factors for abnormal TCD
| Parameters at baseline . | n . | Abnormal TCD . | Normal TCD . | OR (95% CI) . | P . | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD . | n . | Mean ± SD . | n . | ||||
| WBC count, 109/L | 327 | 16.2 ± 5.2 | 54 | 13.4 ± 4.6 | 273 | 1.12 (1.05-1.19) | < .002 |
| Neutrophil count, 109/L | 301 | 6.8 ± 3.4 | 51 | 6.2 ± 3.1 | 250 | 1.06 (0.97-1.16) | .19 |
| Hemoglobin level, g/dL | 332 | 7.4 ± 0.8 | 55 | 8.1 ± 1.2 | 277 | 0.52 (0.38-0.71) | < .001 |
| Hematocrit, % | 308 | 22.4 ± 2.8 | 51 | 24.5 ± 3.8 | 257 | 0.84 (0.76-0.93) | < .001 |
| MCV, flt | 317 | 83.7 ± 6.3 | 53 | 80.2 ± 9.5 | 264 | 1.05 (1.01-1.08) | .01 |
| Reticulocyte count, 109/L | 303 | 344.7 ± 146.0 | 48 | 278.6 ± 105.3 | 255 | 1.004 (1.002-1.007) | .001 |
| LDH level, IU/L | 273 | 1175.6 ± 333.6 | 38 | 957.0 ± 343.5 | 235 | 1.002 (1.001-1.003) | .001 |
| Platelet count, 109/L | 326 | 360.8 ± 134.7 | 53 | 388.2 ± 118.9 | 273 | 0.998 (0.996-1.001) | .15 |
| HbF, % | 301 | 11.4 ± 6.2 | 47 | 11.4 ± 7.9 | 254 | 0.10 (0.96-1.04) | .93 |
| Parameters at baseline . | n . | Abnormal TCD . | Normal TCD . | OR (95% CI) . | P . | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD . | n . | Mean ± SD . | n . | ||||
| WBC count, 109/L | 327 | 16.2 ± 5.2 | 54 | 13.4 ± 4.6 | 273 | 1.12 (1.05-1.19) | < .002 |
| Neutrophil count, 109/L | 301 | 6.8 ± 3.4 | 51 | 6.2 ± 3.1 | 250 | 1.06 (0.97-1.16) | .19 |
| Hemoglobin level, g/dL | 332 | 7.4 ± 0.8 | 55 | 8.1 ± 1.2 | 277 | 0.52 (0.38-0.71) | < .001 |
| Hematocrit, % | 308 | 22.4 ± 2.8 | 51 | 24.5 ± 3.8 | 257 | 0.84 (0.76-0.93) | < .001 |
| MCV, flt | 317 | 83.7 ± 6.3 | 53 | 80.2 ± 9.5 | 264 | 1.05 (1.01-1.08) | .01 |
| Reticulocyte count, 109/L | 303 | 344.7 ± 146.0 | 48 | 278.6 ± 105.3 | 255 | 1.004 (1.002-1.007) | .001 |
| LDH level, IU/L | 273 | 1175.6 ± 333.6 | 38 | 957.0 ± 343.5 | 235 | 1.002 (1.001-1.003) | .001 |
| Platelet count, 109/L | 326 | 360.8 ± 134.7 | 53 | 388.2 ± 118.9 | 273 | 0.998 (0.996-1.001) | .15 |
| HbF, % | 301 | 11.4 ± 6.2 | 47 | 11.4 ± 7.9 | 254 | 0.10 (0.96-1.04) | .93 |
Events are defined as abnormally high velocities. Statistically significant P values are indicated in bold.
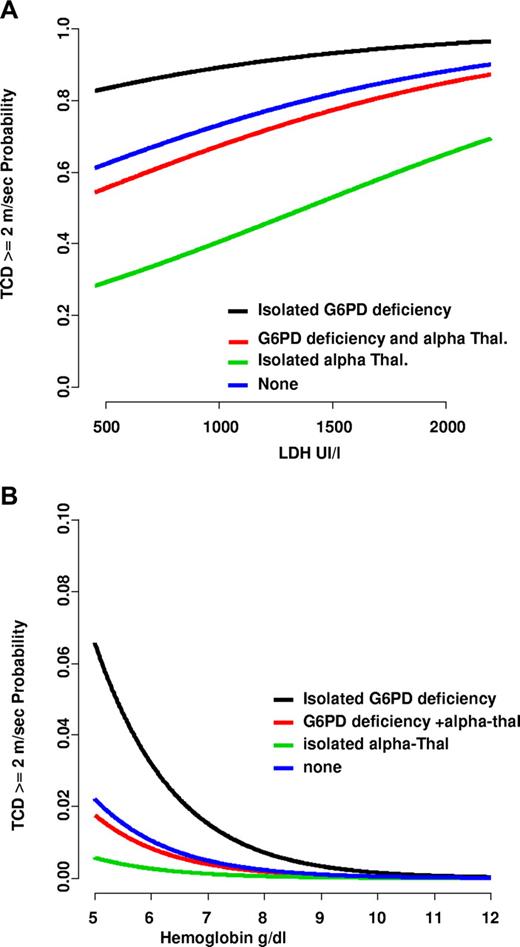
To gain further insight, a multivariate logistic regression model was fitted to assess the set of independent variables contributing additional prognostic information among those selected by the univariable analyses. Of the 9 variables introduced in the model, only G6PD deficiency (odds ratio [OR] = 3.36, 95% confidence interval [CI] 1.10-10.33; P = .034); absence of α-thalassemia (OR = 6.45, 95% CI 2.21-18.87; P = .001); hemoglobin (OR per g/dL = 0.63, 95% CI 0.41-0.97; P = .038); and LDH levels (OR per IU/L = 1.001, 95% CI 1.000-1.002; P = .047) remained as independent predictors of high velocities. Of note, these findings were minimally affected by the recoding of missing values at the median sample values. The risk of abnormal TCD, defined as as a function of LDH (Figure 1A) or Hb (Figure 1B), was maximal in patients with G6PD deficiency and no α-thalassemia (n = 14) and minimal in those with normal G6PD and α-thalassemia (n = 124), while intermediate in others, that is, those with α-thalassemia and G6PD deficiency (n = 17) or those with neither G6PD deficiency nor α-thalassemia (n = 136). Morever, the risk increased for all patients with the severity of anemia but even more with the hemolytic rate (LDH). Actually, predicted probabilities of abnormal TCD were, on average, 0.17 in the subset of patients with normal TCD and 0.49 in those with abnormal TCD.
Predicted probability from the multivariate logistic model of abnormally high velocities. Abnormally high velocities (≥ 2 m/sec) as a function of LDH (A) or hemoglobin level (B) in different patient groups. Patients with isolated α-thalassemia associated with normal G6PD (n = 90) are represented by green lines; patients with isolated G6PD deficiency without α-thalassemia (n = 13) by black lines; and patients with G6PD deficiency and α-thalassemia (n = 114) by red lines. Blue lines represent patients with neither G6PD deficiency nor α-thalassemia. The probability of abnormal TCD increases with low hemoglobin levels and, more importantly, with the degree of hemolysis.
Predicted probability from the multivariate logistic model of abnormally high velocities. Abnormally high velocities (≥ 2 m/sec) as a function of LDH (A) or hemoglobin level (B) in different patient groups. Patients with isolated α-thalassemia associated with normal G6PD (n = 90) are represented by green lines; patients with isolated G6PD deficiency without α-thalassemia (n = 13) by black lines; and patients with G6PD deficiency and α-thalassemia (n = 114) by red lines. Blue lines represent patients with neither G6PD deficiency nor α-thalassemia. The probability of abnormal TCD increases with low hemoglobin levels and, more importantly, with the degree of hemolysis.
This study corroborates previous reports11,12 showing that the risk for stroke in SCA patients is significantly decreased by the presence of α-thalassemia. Abnormal velocities were observed in 22.3% (38/170) patients without α-thalassemia, but only in 7.7% (12/155) patients with α-thalassemia (P < .001). Patients with α-thalassemia had significantly lower WBC count (P = .007), MCV (P < .001), reticulocytes (P = .015), LDH (P = .044), and higher Hb (P = .038) and Hct (P = .003) than patients without. Significance was not lost when adjusting the analysis for Hb, MCV, WBC count and LDH, indicating that the protective effect of α-thalassemia is not simply a marker for higher Hb level and a microcytosis effect, as previously suggested,12 or a marker for lower WBC count and hemolytic rate, as shown here. Although further studies will be required, we suspect that other protective mechanisms, such as improved erythrocyte deformability13 combined with decreased erythrocyte adhesion to vascular endothelial cells due to decreased production of α globins and decreased membrane phosphotidyl-serine exposure,12,14 may be at play.
In addition, we show for the first time that G6PD deficiency is a significant risk factor for high velocities (OR = 3.36). Previous studies had found no evidence that G6PD deficiency associated with SCA increases the severity of hemolysis or the incidence of acute anemic episodes.15 Indeed, the trend toward less severe anemia (7.64 vs 7.97 g/dL) in SCA patients with G6PD deficiency (SCA/G6PD) was not significant (P = .19) in our study. In addition, the biologic parameters (WBC, PMN, Hb, Hct, reticulocytes, MCV, LDH, and HbF) were not significantly different between SCA and SCA/G6PD patients. Altogether, this suggests that G6PD deficiency increases risk by a mechanism other than hemolysis. G6PD has long been considered as an antioxidant enzyme in erythrocytes.16 Accumulating data now indicate that G6PD has a similar role in vascular cells under conditions of increased production of reactive oxygen species (ROS).17 Manipulating G6PD levels in cultured cells and animal models demonstrated that decreased G6PD expression correlates with increased ROS accumulation and oxidative cell injury in endothelial cells, suggesting a significant role for G6PD in modulating vascular redox state.18 Furthermore, G6PD deficiency causes a decrease in endothelial nitric oxide (NO·) bioavailability,19 which contributes to vascular dysfunction. Given that SCA is also associated with increased ROS production and impaired NO· metabolism,20,21 SCA redox-dependent events that lead to vascular pathology may be compounded by G6PD deficiency.
The role of hemolysis in the pathophysiology of SCA has recently been reexamined, in view of new evidence demonstrating the effects of hemolysis on NO· homeostasis. Hemolysis releases hemoglobin, which consumes NO·. Furthermore, heme and heme iron dissociate from hemoglobin and catalyze the production of ROS, which are potent NO· scavengers.22 Hemolytic rate, reported by LDH, correlates closely with NO· consumption23 and with prevalence and severity of pulmonary hypertension (PHT),24 suggesting a critical role for dysregulated NO· metabolism in PHT. However, the link between hemolysis and stroke is not entirely clear.24 A recent report showed a significant positive correlation between LDH and TCD velocities.25 Our findings that LDH is an independent predictor for high velocities suggest that cerebral macrovasculopathy, like PHT, may be linked to hemolysis and NO· bioavailability.
In summary, this study confirms the protective role of α-thalassemia against cerebral vasculopathy and shows that G6PD deficiency, anemia and hemolysis are significant and independent risk factors for stroke, using TCD as a surrogate marker. These findings further extend our understanding of the modifying factors that contribute to cerebral vasculopathy in SCA.
Presented in part at the 49th annual meeting of the American Society of Hematology, Atlanta, December 10, 2007.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.B. designed the research, analyzed data, and wrote the paper; S.V. performed TCD and analyzed data; S.C. performed statistical analysis; M.T. contributed to the writing of the paper; L.C., C.A., A.K., I.H., C.D., and F.B. were involved in the care of patients; and M.G.N. performed α and β genes study. All authors participated in the review of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Bernaudin, Centre de Référence de Drépanocytose, Hôpital Intercommunal de Créteil, 40 avenue de Verdun, 94010, Créteil, France; e-mail: francoise.bernaudin@chicreteil.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal