Abstract
Myeloid-derived suppressor cells (MDSCs) accumulate in cancer patients and tumor-bearing mice and potently suppress T-cell activation. In this study, we investigated whether MDSCs regu-late natural killer (NK)–cell function. We discovered that mononuclear Gr-1+CD11b+F4/80+ MDSCs isolated from RMA-S tumor-bearing mice do not suppress, but activate NK cells to produce high amounts of IFN-γ. Gr-1+CD11b+F4/80+ MDSCs isolated from tumor-bearing mice, but not myeloid cells from naive mice, expressed the ligand for the activating receptor NKG2D, RAE-1. NK-cell activation by MDSCs depended partially on the interaction of NKG2D on NK cells with RAE-1 on MDSCs. NK cells eliminated Gr-1+CD11b+F4/80+ MDSCs in vitro and upon adoptive transfer in vivo. Finally, depletion of Gr-1+ cells that comprise MDSCs confirmed their protective role against the NK-sensitive RMA-S lymphoma in vivo. Our study reveals that MDSCs do not suppress all aspects of antitumor immune responses and defines a novel, unexpected activating role of MDSCs on NK cells. Thus, our results have great impact on the design of immune therapies against cancer aiming at the manipulation of MDSCs.
Introduction
Effective antitumor immune responses require a close collaboration between armed effector cells of the innate and the adaptive immune system. The success of immune responses against tumors is often limited due to multiple regulatory mechanisms, including an immune suppressive tumor environment and an expansion of suppressive cell types. It is well established that regulatory T cells (Tregs) inhibit antitumor immune responses in vitro and in vivo. Furthermore, immature myeloid cells were described to accumulate in cancer patients and tumor-bearing mice and to suppress the proliferation and cytokine production of T cells (reviewed in Serafini et al1 and Sica and Bronte2 ). These cells were recently defined as myeloid-derived suppressor cells (MDSCs).3
MDSCs are characterized by a combination of phenotypical and functional properties. With regards to their phenotype, mouse MDSCs represent a heterogeneous population of immature myeloid cells expressing CD11b and Gr-1. Additional markers, including F4/80, CD31, or CD115, were reported to more precisely define the MDSC subpopulation with suppressive function on T cells.4-6 Recently, 2 subpopulations within Gr-1+CD11b+ MDSCs were described.7 Ly6G+ with high side light scattering properties (SSChigh) and Ly6G− with low side light scattering properties (SSClow) MDSCs were designated as polymorphonuclear MDSCs (PMN-MDSCs) and mononuclear MDSCs (MO-MDSCs), respectively.7 The majority of the MO-MDSCs expressed the monocyte/macrophage marker F4/80. A small number of cells expressing CD11b and Gr-1 is also detectable in blood and spleens of naive mice.8-10 However, this cell population isolated from naive mice suppresses T-cell proliferation to a much lesser extent.8,9 So far, all surface markers proposed for the phenotypical characterization of MDSCs are not only expressed by MDSCs but are also present on other cell types in naive or tumor-bearing mice. A marker exclusively expressed on MDSCs has not been defined.
It is well established that MDSCs potently suppress antigen or ConA-induced T-cell proliferation and production of IFN-γ and IL-2.9,11-13 The mechanisms of this inhibition are not completely understood. It was reported that nitric oxide (NO) produced by MDSCs inhibits T-cell proliferation.14,15 Furthermore, MDSCs suppress T-cell proliferation by down-regulation of surface expression of the CD3ζ chain via reactive oxygen species (ROS).16 Recently, Nagaraj et al12 demonstrated that ROS and peroxynitrite produced by MDSCs inhibit CD8+ T-cell proliferation by interfering with TCR-mediated recognition of peptide-loaded MHC class I molecules.
Natural killer (NK) cells, which are cytolytic and cytokine-producing effector cells, serve as a first line of immune defense against tumors. Their activation is determined by a delicate balance between inhibitory receptors, most of which are specific for self-MHC class I molecules, and activating receptors.17 The activating receptor NKG2D, which in mice recognizes RAE-1, H60, and MULT1,18-20 plays an important role in the immune response against cancer.21-23 Its ligands are rarely expressed on the cell surface of healthy cells and tissues but frequently on tumors and tumor cell lines.19,20,24 Furthermore, NK-cell activation is controlled by additional factors. In this context, several studies demonstrated that NK cells are efficiently activated by different types of myeloid cells. In humans, cytotoxic function and IFN-γ secretion by NK cells are induced by immature and mature dendritic cells (DCs).25,26 Furthermore, it was reported that adoptively transferred DCs promote NK cell–dependent antitumor responses in a MHC class I–negative tumor model.27 Human NK cells also differentiate and/or kill immature DCs isolated from healthy individuals.25,28,29 Moreover, human NK cells were shown to kill LPS-stimulated macrophages via NKG2D.30
So far, little is known about the cross-talk of NK cells with myeloid cells isolated from tumor patients or tumor-bearing mice. In this context, Liu et al31 reported that Gr-1+CD11b+ MDSCs isolated from tumor-bearing mice inhibited IL-2–induced NK cell–mediated killing of YAC-1 targets, which correlated with an inhibition of STAT5 activation in NK cells. In another study, IFN-β–induced NK cell–mediated cytotoxicity was suppressed by MDSCs.32 Currently, it is not known whether all NK-cell activation pathways and effector functions are modulated by MDSCs. Whether NK cells also regulate MDSC function has not yet been addressed.
Our study aims at defining and eliminating the mechanisms counteracting NK-cell activation, in order to amplify NK cell–mediated antitumor immune responses in vivo. To this end, we investigated the cross-talk of NK cells and MDSCs in the NK cell–sensitive tumor model RMA-S. We demonstrate that Gr-1+CD11b+F4/80+ MDSCs, which accumulate in RMA-S tumor-bearing mice, potently inhibit T-cell proliferation. Importantly, Gr-1+CD11b+F4/80+ MDSCs expressed the NKG2D ligand RAE-1 and activated NK cells to produce large amounts of IFN-γ that were induced, in part, via the NKG2D activating receptor. Furthermore, NK cells eliminated Gr-1+CD11b+F4/80+ cells in vitro and in vivo after adoptive transfer. Depletion of Gr-1+ cells that comprise MDSCs led to enhanced RMA-S tumor growth. In summary, our data reveal an unexpected multifaceted role of MDSCs in antitumor immune responses, including the activation of NK-cell effector functions.
Methods
Mice
C57BL/6 and C57BL/6-Ly5.1 mice were purchased from Charles River Laboratories (Sulzfeld, Germany and Brussels, Belgium, respectively). IFN-γ−/− C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed under specific pathogen-free conditions and in accordance with all standards of animal care. All animal experiments were approved by the Regierungspräsidium Karlsruhe.
Tumor model and in vivo antibody treatment
MHC class I–deficient RMA-S or MHC class I–expressing RMA lymphoma cells33 were cultured in RPMI-1640 medium, 10% fetal calf serum (FCS), 1% l-glutamine, 1% penicillin, and 1% streptomycin (Invitrogen, Karlsruhe, Germany). For tumor cell injection, cells were washed at least 3 times with phosphate-buffered saline (PBS). RMA-S (1 × 106) or RMA (1 × 105) were injected subcutaneously in 100 μL PBS in the shaved left flank of 7- to 8-week-old female mice. Tumor growth was monitored by determining the tumor volume every second or third day by using a caliper. For the in vivo neutralization of IFN-γ, 300 μg anti–mouse IFN-γ mAb (clone XMG1.2; BioExpress, Kaysville, UT) were injected intraperitoneally 3 times weekly beginning at the day of tumor cell inoculation.
To deplete NK1.1+ cells, 200 μg anti-NK1.1 mAb (clone PK136; BioExpress) was injected intraperitoneally on days −2, +2, +9, and +16 of tumor cell inoculation. For depletion of Gr-1+ cells, 300 μg of an anti–Gr-1 mAb (clone RB6–8C5; BioExpress) or an isotype-matched control mAb (clone LTF-2) were injected intraperitoneally on days −2, +2, and subsequently every third day of tumor cell inoculation.
Cell preparation, mAbs, and flow cytometry
For the characterization of different cell types by flow cytometry the following antibodies were used (all purchased from BD Biosciences, San Jose, CA): APC-conjugated anti-Gr-1 (clone RB6-8C5), FITC-conjugated anti-CD11b (clone M1/70), PE-conjugated anti-CD124 (clone mIL4R-M1), FITC-conjugated anti-Ly6G (clone 1A8), and PerCp-Cy5.5–conjugated anti-Ly5.2 (clone 104). PE-conjugated anti-F4/80 or Alexa488-conjugated anti-F4/80 mAbs were obtained from Invitrogen and PE-conjugated anti-panRAE-1 (186107) was purchased from R&D Systems (Minneapolis, MN), respectively. For flow cytometric analysis of peripheral blood leukocytes (PBLs), blood was collected and red cell lysis was performed after cell labeling using the BD FACS Lysing Solution (BD Biosciences). Stainings were performed in PBS, 10% Fc block (supernatant of the anti-CD16/CD32 hybridoma, clone 2.4G2) and 0.02% sodium azide. Stained cells were analyzed on a FACSCalibur using the CellQuestPro Software (BD Biosciences). For all cell purifications from blood and spleens by FACS sorting, erythrocyte lysis was performed using a buffer containing 150 mM NH4Cl, 100 mM KHCO3, and 0.1 mM EDTA, pH = 7.3. NK cells were isolated from splenocytes by using magnetic beads coated with DX5 mAb (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of NK1.1+ cells was greater than 80%.
In most experiments purification of Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− was performed by flow cytometric sorting. Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− were isolated from tumor-bearing mice on day 20 or 21 after tumor cell inoculation. Single cell suspensions were stained with anti–Gr-1 and anti-F4/80 mAbs. Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− fractions were sorted by using a FACSDiva (BD Biosciences). Purities of Gr-1+CD11b+F4/80− cells were greater than 95% and of Gr-1+CD11b+F4/80+ cells greater than 80%, respectively (Figure S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Contaminating cells within the Gr-1+CD11b+F4/80+ fraction were mainly Gr-1+CD11b+F4/80− cells. To obtain tumor-infiltrating cells, tumors were removed, cut into small pieces, and digested with 5 mg/mL collagenase type IV (Cell Systems, St Katharinen, Germany) and 0.5 mg/mL DNase I (Sigma-Aldrich, Seelze, Germany) at 37°C for 15 minutes. Subsequently, erythrocytes were lysed and cells were sorted using flow cytometry. To obtain enriched Gr-1+CD11b+F4/80− and Gr-1+CD11b+F4/80+ cells, a method using magnetic beads was applied. For this purpose, Ly6G+ cells from blood or spleen cells were positively selected using an anti-Ly6G isolation kit (Miltenyi Biotec). These cells represent Gr-1+CD11b+F4/80− cells and were obtained at a purity greater than 85%. Ly6G− cells collected from the Ly6G isolation were stained with an APC-conjugated anti–Gr-1 mAb and sorted using anti-APC microbeads (Miltenyi Biotec). This population represents Gr-1+CD11b+F4/80+ cells and were obtained at a purity greater than 65%.
Proliferation assay
Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from spleens of tumor-bearing mice were sorted by flow cytometry and cocultured with 1 × 105 splenocytes from naive C57BL/6 mice with ConA (2 μg/mL; Sigma-Aldrich) at the indicated ratios. In some experiments, L-NMMA (0.5 mM) or D-NMMA (0.5 mM; Alexis, Lausen, Switzerland) was added to the cultures. Cells were cultured for 72 hours and 1 μCi [37 kBq] 3H-thymidine (GE Healthcare, Little Chalfont, United Kingdom) was added for the final 18 hours of culture. Cells were harvested, and 3H-thymidine incorporation was measured with a MicroBeta TriLux Counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). To determine NK-cell proliferation, 1 × 105 purified NK cells were stimulated with 400 U/mL human IL-2, and cultured at the indicated ratios with isolated Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells for 48 hours. The proliferation rate was determined by a standard 3H-thymidine incorporation assay. All cultures of primary cells were performed in RPMI-1640 medium, 10% FCS, 1% l-glutamine, 1% penicillin, and 1% streptomycin, 100 mM nonessential amino acids, 100 mM sodium-pyruvate, and 50 mM β-mercaptoethanol (Invitrogen) in 96-well round-bottom microplates.
Analysis of IFN-γ production
NK cells were purified from naive C57BL/6 or B6 IFN-γ−/− mice. Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice were sorted by flow cytometry. NK cells (1 × 105) were cultured with Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− at different ratios in the absence or presence of 2 ng/mL IL-12. Supernatants were harvested after 24 hours and analyzed for IFN-γ production by specific enzyme-linked immunosorbent assay (ELISA; BD Biosciences). In some experiments, transwell plates (Fisher Scientific, Schwerte, Germany) were used. Fab fragments of an anti-NKG2D mAb (clone 191004; R&D Systems) or of an appropriate isotype-matched control mAb (clone 141945) were generated with a Fab preparation kit (Pierce, Rockford, IL). Subsequently, the buffer was replaced by PBS using PD10 columns (GE Healthcare). The quality of purified Fab fragments was verified on SDS-PAGE and Coomassie Blue staining. Fab fragments of the anti-NKG2D mAb or an isotype-matched control mAb were used for blocking experiments at a concentration of 10 μg/mL. For transwell and blocking experiments MACS-sorted Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− of blood of tumor-bearing mice were used.
Cytotoxicity assay
NK cells were purified from naive mice and cocultured with MACS-sorted Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells for 4 hours. Cells were washed, percentages of NK cells were determined by flow cytometry, and NK-cell numbers were adjusted to obtain similar NK-cell numbers in the cytotoxicity assay. Subsequently, NK cells were used as effector cells against YAC-1 target cells at different ratios in a standard 4-hour 51Cr-release assay.
In vitro killing
NK cells were isolated from naive mice and expanded in 1700 U/mL human IL-2 for 5 to 10 days. Gr-1+ cells were isolated from RMA-S tumor-bearing mice. PBLs were labeled with APC-conjugated anti–Gr-1 mAb and enriched with anti-APC magnetic microbeads (Miltenyi Biotec). Gr-1+ cells were cocultured with IL-2–expanded NK cells at different effector target ratios for 12 hours. Subsequently, cells were stained with mAbs specific for Gr-1 and F4/80. In addition, cells were stained with an anti–NK1.1 mAb to exclude NK cells from the analysis by gating. Cells were analyzed by flow cytometry, and the percentages of Gr-1+CD11b+F4/80+ among Gr-1+ cells were determined.
Adoptive transfer of NK cells
NK cells were purified from naive mice (C57BL/6-Ly5.2) and washed 3 times with PBS. NK cells (4-6 × 106) were injected intravenously into tumor-bearing C57BL/6-Ly5.1 mice at day 18 or 19 after tumor cell inoculation. “Mock”-treated mice were bled at similar time points, but did not receive NK cells. Percentages of Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells among total PBLs were determined by flow cytometry 4 hours before and 5 hours after transfer of NK cells. Adoptively transferred NK cells were excluded by gating on Ly5.2-negative cells.
Results
Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− MDSCs accumulate in RMA-S tumor-bearing mice
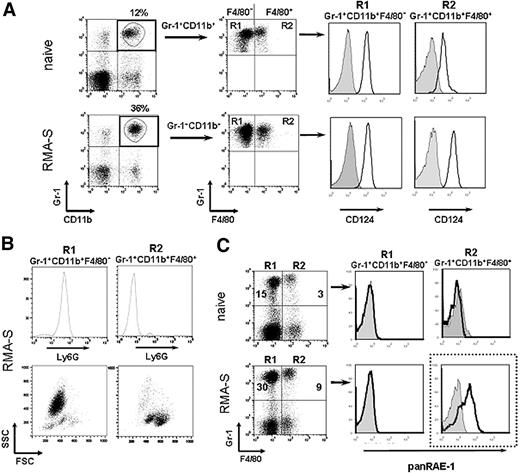
Previously, most studies that investigated the role of MDSCs in tumor models have focused on the interactions between MDSCs and T cells.8,34,35 The goal of our study was to determine the role of MDSC in NK cell–sensitive tumor models. MHC class I–deficient RMA-S cells, when injected at relatively low cell numbers, are rejected by NK cells.33 In our study, we injected a higher cell number of RMA-S cells subcutaneously that led to progressive tumor growth over a period of 3 weeks. As described for other tumor models, we also observed an accumulation of Gr-1+CD11b+ cells in blood and spleens of tumor-bearing mice as compared with naive mice (Figure 1A and data not shown). Gr-1+CD11b+ cells represent a heterogeneous population, including cells of granulocytic and monocytic origin.7 Therefore, we further characterized Gr-1+CD11b+ cells by the expression of the monocytic marker F4/80 that divides Gr-1+CD11b+ cells into 2 subsets, Gr-1+CD11b+F4/80− and Gr-1+CD11b+F4/80+ cells. Gr-1+CD11b+F4/80+ from tumor-bearing mice expressed higher levels of CD124 (IL-4Rα) as compared with naive mice (Figure 1A). Gr-1+CD11b+F4/80− cells expressed Ly6G and intermediate levels of Ly6C. In contrast, Gr-1+CD11b+F4/80+ did not express Ly6G, but did express high levels of Ly6C (Figure 1B and data not shown). These subsets were recently designated as polymorphonuclear MDSCs (PMN-MDSCs) or mononuclear MDSCs (MO-MDSCs), respectively.7 An analysis of the 2 subsets by forward and side scatter (FSC/SSC) profile revealed that Gr-1+CD11b+F4/80− cells were mainly SSChigh and Gr-1+CD11b+F4/80+ were SSClow (Figure 1B). Of note, percentages of both Gr-1+CD11b+F4/80− and Gr-1+CD11b+F4/80+ were elevated in tumor-bearing mice as compared with naive mice (Figure 1C). Also, total cell numbers of Gr-1+CD11b+F4/80− and Gr-1+CD11b+F4/80+ cells significantly increased in the blood with progressive tumor growth (Figure S1A and data not shown). In order to extend our finding to other tumor models, we also determined the numbers of Gr-1+F4/80+ cells after subcutaneous inoculation of MHC class I–expressing RMA lymphoma cells transduced with the NKG2D ligand RAE-1γ (RMA-RAE-1γ) and B16BL6 melanoma cells. Also in these tumor models, we found a significant accumulation of Gr-1+CD11b+F4/80+ cells in the blood of tumor-bearing mice (Figure S1B).
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice express RAE-1 and suppress T cell proliferation. (A) Peripheral blood leukocytes (PBLs) of naive and tumor-bearing mice isolated on day 21 after tumor cell inoculation were stained with mAbs specific for Gr-1 and CD11b in combination with mAbs directed against F4/80 and CD124 (IL-4Rα). Plots are gated on Gr-1+CD11b+ cells. Histograms were further gated on Gr-1+CD11b+F4/80− (R1) or Gr-1+CD11b+F4/80+ (R2) cells to analyze the expression of CD124 (solid lines) compared with the respective isotype-matched control Ig (gray filled histograms). Flow cytometric analysis of PBLs of one representative of 5 animals per group is shown. (B) PBLs from tumor-bearing mice were gated on Gr-1+CD11b+F4/80− (R1) or Gr-1+CD11b+F4/80+ (R2) as described above and analyzed for the expression of Ly6G. Gated cells are also depicted in a forward and side scatter plot. (C) PBLs from naive and tumor-bearing mice (day 21) were stained with mAbs specific for Gr-1 and F4/80 in combination with a mAb directed against panRAE-1 (solid lines) or the respective isotype-matched Ig controls (gray filled histograms). Histograms are gated on Gr-1+F4/80− (R1) or Gr-1+F4/80+ (R2). For R1 and R2, percentages among all PBLs are indicated in the plots. (A-C) One representative experiment of at least 3 independent experiments is shown.
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice express RAE-1 and suppress T cell proliferation. (A) Peripheral blood leukocytes (PBLs) of naive and tumor-bearing mice isolated on day 21 after tumor cell inoculation were stained with mAbs specific for Gr-1 and CD11b in combination with mAbs directed against F4/80 and CD124 (IL-4Rα). Plots are gated on Gr-1+CD11b+ cells. Histograms were further gated on Gr-1+CD11b+F4/80− (R1) or Gr-1+CD11b+F4/80+ (R2) cells to analyze the expression of CD124 (solid lines) compared with the respective isotype-matched control Ig (gray filled histograms). Flow cytometric analysis of PBLs of one representative of 5 animals per group is shown. (B) PBLs from tumor-bearing mice were gated on Gr-1+CD11b+F4/80− (R1) or Gr-1+CD11b+F4/80+ (R2) as described above and analyzed for the expression of Ly6G. Gated cells are also depicted in a forward and side scatter plot. (C) PBLs from naive and tumor-bearing mice (day 21) were stained with mAbs specific for Gr-1 and F4/80 in combination with a mAb directed against panRAE-1 (solid lines) or the respective isotype-matched Ig controls (gray filled histograms). Histograms are gated on Gr-1+F4/80− (R1) or Gr-1+F4/80+ (R2). For R1 and R2, percentages among all PBLs are indicated in the plots. (A-C) One representative experiment of at least 3 independent experiments is shown.
Gr-1+CD11b+F4/80+ cells isolated from RMA-S tumor-bearing mice express the NKG2D ligand RAE-1
Because the aim of our study was to investigate the cross-talk between MDSCs and NK cells, we stained MDSCs for the expression of surface molecules involved in NK cell recognition. Importantly, we observed that Gr-1+CD11b+F4/80+ cells isolated from tumor-bearing mice, but not from naive mice, expressed RAE-1, a ligand for the activating receptor NKG2D (Figure 1C). In contrast, Gr-1+CD11b+F4/80− cells from tumor-bearing and naive mice did not express RAE-1 (Figure 1C). Incubation with an unlabeled mAb specific for RAE-1ϵ prior to staining abrogated staining with the directly conjugated anti–panRAE-1 mAb (data not shown). No other cell population in the blood of naive or tumor-bearing mice stained with mAbs directed against RAE-1 (data not shown), indicating that RAE-1 was exclusively expressed on F4/80+ MDSCs isolated from tumor-bearing mice. In order to investigate whether RAE-1 was also expressed on MDSCs in other tumor models, we determined RAE-1 expression on Gr-1+CD11b+F4/80+ cells from RMA-RAE-1γ lymphoma- or B16BL6 melanoma-bearing mice. Indeed, Gr-1+CD11b+F4/80+ cells, which accumulated in the blood of tumor-bearing mice, specifically expressed RAE-1 (Figure S1C). Thus, our data show for the first time that Gr-1+ CD11b+F4/80+ cells from tumor-bearing mice, but not from naive mice, are characterized by the expression of the NKG2D ligand RAE-1.
Gr-1+CD11b+F4/80+ from RMA-S tumor-bearing mice suppress T-cell proliferation
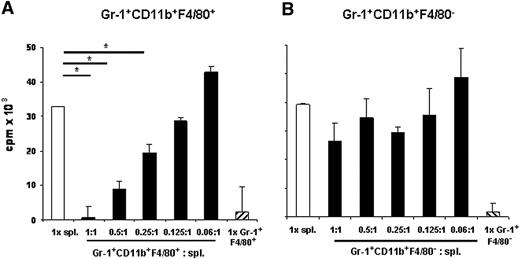
Next, we examined whether Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice functionally resembled MDSCs and suppressed T-cell proliferation. Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− isolated from spleens of tumor-bearing mice were sorted by flow cytometry. Expression of CD11b on sorted cells was confirmed by flow cytometric analysis (data not shown). Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells were cultured in the absence or in the presence of splenocytes and ConA at the indicated ratios and proliferation was determined. Importantly, Gr-1+CD11b+F4/80+ cells potently suppressed T-cell proliferation (Figure 2A), whereas Gr-1+CD11b+F4/80− only had a minor effect (Figure 2B). Similar results were obtained with Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− isolated from PBLs of tumor-bearing mice (data not shown).
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice suppress T-cell proliferation. Sorted Gr-1+CD11b+F4/80+ (A) or Gr-1+CD11b+F4/80− (B) cells isolated from tumor-bearing mice on day 21 after tumor cell inoculation were cocultured with splenocytes (spl) from naive mice for 72 hours with ConA at the indicated ratios. Proliferation was assessed by 3H-thymidine incorporation assay. Data show mean plus or minus standard deviation (SD) of triplicate cultures. Asterisks indicate statistical significance (P < .01) determined by Student t test. Representative data selected from 3 independent experiments are shown.
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice suppress T-cell proliferation. Sorted Gr-1+CD11b+F4/80+ (A) or Gr-1+CD11b+F4/80− (B) cells isolated from tumor-bearing mice on day 21 after tumor cell inoculation were cocultured with splenocytes (spl) from naive mice for 72 hours with ConA at the indicated ratios. Proliferation was assessed by 3H-thymidine incorporation assay. Data show mean plus or minus standard deviation (SD) of triplicate cultures. Asterisks indicate statistical significance (P < .01) determined by Student t test. Representative data selected from 3 independent experiments are shown.
MDSCs do not affect proliferation and cytotoxic activity of NK cells
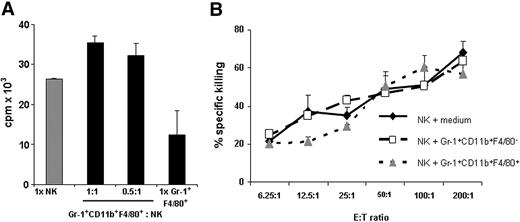
We investigated whether Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice also suppressed the proliferation of NK cells. NK cells isolated from naive mice were cultured with IL-2 (400 U/mL) in the absence or presence of Gr-1+CD11b+F4/80+ cells at the indicated ratios. The addition of Gr-1+CD11b+F4/80+ cells did not inhibit, but slightly increased, proliferation in these cocultures compared with NK cells cultured alone (Figure 3A). This small increase in proliferation is likely due to the low levels of proliferation of Gr-1+CD11b+F4/80+ cells cultured by themselves. Gr-1+CD11b+F4/80− cells from tumor-bearing mice also did not suppress NK cell proliferation (data not shown). In order to investigate whether Gr-1+CD11b+F4/80+ cells affected NK cell–mediated killing, the cytolytic activity of NK cells cultured in the absence or presence of Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice against YAC-1 targets was determined. Neither population suppressed NK cell–mediated lysis of YAC-1 targets (Figure 3B). We conclude that under our experimental conditions the in vitro proliferation and cytotoxic activity of NK cells is not adversely affected by MDSCs from RMA-S tumor-bearing mice.
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice do not affect NK cell proliferation and cytotoxic activity. (A) NK cells isolated from naive mice were cultured with 400 U/mL IL-2 for 48 hours in the absence or presence of Gr-1+CD11b+F4/80+ cells from tumor-bearing mice at the indicated ratios. Proliferation was determined by 3H-thymidine incorporation assay. (B) Purified Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice were cultured with NK cells from naive mice for 4 hours and used as effector cells against YAC-1 targets at the indicated ratios. Specific killing was determined by a standard 4-hour 51Cr-release assay. Data represent mean plus or minus SD of triplicate cultures. (A,B) One representative experiment of 2 independent experiments is shown.
Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice do not affect NK cell proliferation and cytotoxic activity. (A) NK cells isolated from naive mice were cultured with 400 U/mL IL-2 for 48 hours in the absence or presence of Gr-1+CD11b+F4/80+ cells from tumor-bearing mice at the indicated ratios. Proliferation was determined by 3H-thymidine incorporation assay. (B) Purified Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice were cultured with NK cells from naive mice for 4 hours and used as effector cells against YAC-1 targets at the indicated ratios. Specific killing was determined by a standard 4-hour 51Cr-release assay. Data represent mean plus or minus SD of triplicate cultures. (A,B) One representative experiment of 2 independent experiments is shown.
MDSCs activate NK cells to produce high amounts of IFN-γ
MDSCs were reported to inhibit antigen-specific IFN-γ production by CD8+ T cells.9,12,36,37 To determine the effect of MDSCs on IFN-γ production by NK cells, NK cells from naive mice were cultured in the absence or presence of Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice and IFN-γ production was measured. NK cells alone did not produce significant amounts of IFN-γ. Importantly, NK cells cocultured with Gr-1+CD11b+F4/80+ from tumor-bearing mice produced substantial amounts of IFN-γ (Figure 4A left panel). In contrast, Gr-1+CD11b+F4/80− cells did not induce IFN-γ production by NK cells (Figure 4A right panel). In the presence of IL-12, NK cells produced larger amounts of IFN-γ. When NK cells were cocultured with Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice in the presence of IL-12, highly elevated amounts of IFN-γ were detected (Figure 4B left panel). On the contrary, in cocultures with Gr-1+CD11b+F4/80− cells, IFN-γ production was only slightly enhanced (Figure 4B right panel). Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells cultured alone did not produce significant amounts of IFN-γ. When Gr-1+CD11b+F4/80+ cells sorted from naive mice were cocultured with NK cells, lower amounts of IFN-γ were induced (data not shown). In addition, Gr-1+CD11b+F4/80+ cells isolated from tumors also induced the production of IFN-γ, indicating that F4/80+ MDSCs also activate NK cells locally within the tumor (Figure 4C). Tumor-infiltrating Gr-1+CD11b+F4/80− cells enhanced the production of IFN-γ by NK cells to a lesser extent (data not shown). To investigate whether IFN-γ was released by NK cells or by MDSCs, we measured IFN-γ secretion in cocultures of Gr-1+CD11b+F4/80+ from RMA-S tumor-bearing wild-type mice with NK cells isolated from wild-type or IFN-γ−/− mice, respectively. High amounts of IFN-γ were detected when NK cells originated from wild-type mice, but no IFN-γ was detectable when NK cells were isolated from IFN-γ−/− mice (Figure 4D). Therefore, NK cells are the main source of IFN-γ. In summary, our data indicate that MDSCs are potent inducers of IFN-γ production by NK cells.
NK cells cocultured with Gr-1+CD11b+F4/80+ cells produce large amounts of IFN-γ. (A-D) Purified Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice were cocultured with NK cells at the indicated ratios. Supernatants were harvested after 24 hours and IFN-γ amounts were determined by ELISA. (A) NK cells from naive mice were cultured alone or in the presence of Gr-1+CD11b+F4/80+ cells (left panel) or Gr-1+CD11b+F4/80− (right panel) in the absence of IL-12. (B) NK cells were cultured in IL-12 in the absence or in the presence of Gr-1+CD11b+F4/80+ (left panel) or Gr-1+CD11b+F4/80− (right panel) cells at the indicated ratios. (C) Tumor-infiltrating Gr-1+CD11b+F4/80+ cells were purified and cultured with NK cells in the presence of IL-12. (D) NK cells isolated from C57BL/6 wt or IFN-γ−/− mice were cultured in IL-12 in the absence of presence of Gr-1+CD11b+F4/80+ cells. Data depict mean plus or minus SD of triplicate cultures and represent one of 2 (A,C,D) or one of 5 (B) independent experiments. Asterisks indicate statistical significance (P < .01) determined by Student t test. ND indicates not detectable.
NK cells cocultured with Gr-1+CD11b+F4/80+ cells produce large amounts of IFN-γ. (A-D) Purified Gr-1+CD11b+F4/80+ or Gr-1+CD11b+F4/80− cells from tumor-bearing mice were cocultured with NK cells at the indicated ratios. Supernatants were harvested after 24 hours and IFN-γ amounts were determined by ELISA. (A) NK cells from naive mice were cultured alone or in the presence of Gr-1+CD11b+F4/80+ cells (left panel) or Gr-1+CD11b+F4/80− (right panel) in the absence of IL-12. (B) NK cells were cultured in IL-12 in the absence or in the presence of Gr-1+CD11b+F4/80+ (left panel) or Gr-1+CD11b+F4/80− (right panel) cells at the indicated ratios. (C) Tumor-infiltrating Gr-1+CD11b+F4/80+ cells were purified and cultured with NK cells in the presence of IL-12. (D) NK cells isolated from C57BL/6 wt or IFN-γ−/− mice were cultured in IL-12 in the absence of presence of Gr-1+CD11b+F4/80+ cells. Data depict mean plus or minus SD of triplicate cultures and represent one of 2 (A,C,D) or one of 5 (B) independent experiments. Asterisks indicate statistical significance (P < .01) determined by Student t test. ND indicates not detectable.
Induction of IFN-γ is NO independent and partially mediated by NKG2D
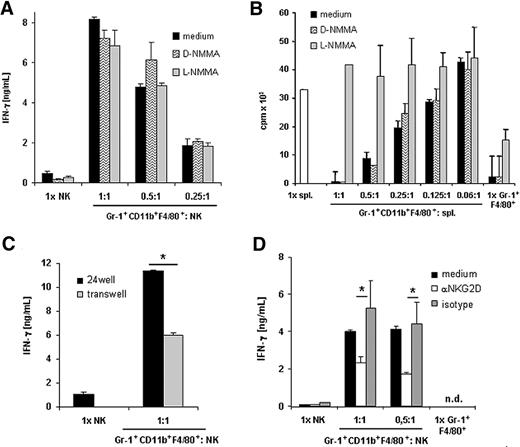
We investigated the mechanism responsible for the induction of IFN-γ in cocultures of NK cells and Gr-1+CD11b+F4/80+ cells from RMA-S tumor-bearing mice. NO is required for IL-12–induced IFN-γ production by NK cells during Leishmania major infection.38 To determine the role of NO, we added the NO inhibitor, L-NG-monomethyl arginine (L-NMMA), or its stereoisomeric inactive isoform D-NMMA to our cocultures. Addition of L-NMMA to the cocultures of Gr-1+CD11b+F4/80+ and NK cells did not inhibit the production of IFN-γ, indicating that in our model induction of IFN-γ production by NK cells is independent of NO (Figure 5A).
Induction of IFN-γ production is NO independent and is partially mediated by NKG2D. (A) NK cells purified from naive mice were cultured in IL-12 in the absence or presence of Gr-1+CD11b+F4/80+ cells isolated from RMA-S tumor bearing mice at the indicated ratios. As indicated, L-NMMA, D-NMMA, or medium only were added. Supernatants were harvested after 24 hours and IFN-γ production was determined by ELISA. (B) Gr-1+CD11b+F4/80+ cells were purified from RMA-S tumor-bearing mice. Gr-1+CD11b+F4/80+ cells were cultured alone or cocultured with splenocytes (spl.) from naive C57BL/6 mice with ConA in the absence or presence of the NO inhibitor L-NMMA or its inactive isoform D-NMMA at the indicated ratios for 72 hours. Proliferation was determined by 3H-thymidine incorporation assay. (C) NK cells were cultured in IL-12 in the absence or presence of Gr-1+CD11b+F4/80+ cells from tumor-bearing mice at a 1:1 ratio using a standard plate or a transwell system. IFN-γ production was determined by ELISA. (D) Gr-1+CD11b+F4/80+ cells were sorted from PBL of tumor-bearing mice. Fab fragments of the anti-NKG2D mAb or of an isotype-matched control mAb were added to cocultures of NK cells and Gr-1+CD11b+F4/80+ cells. Data are shown as mean plus or minus SD of triplicates. One representative experiment of 3 experiments is shown. *P < .05. ND indicates not detectable.
Induction of IFN-γ production is NO independent and is partially mediated by NKG2D. (A) NK cells purified from naive mice were cultured in IL-12 in the absence or presence of Gr-1+CD11b+F4/80+ cells isolated from RMA-S tumor bearing mice at the indicated ratios. As indicated, L-NMMA, D-NMMA, or medium only were added. Supernatants were harvested after 24 hours and IFN-γ production was determined by ELISA. (B) Gr-1+CD11b+F4/80+ cells were purified from RMA-S tumor-bearing mice. Gr-1+CD11b+F4/80+ cells were cultured alone or cocultured with splenocytes (spl.) from naive C57BL/6 mice with ConA in the absence or presence of the NO inhibitor L-NMMA or its inactive isoform D-NMMA at the indicated ratios for 72 hours. Proliferation was determined by 3H-thymidine incorporation assay. (C) NK cells were cultured in IL-12 in the absence or presence of Gr-1+CD11b+F4/80+ cells from tumor-bearing mice at a 1:1 ratio using a standard plate or a transwell system. IFN-γ production was determined by ELISA. (D) Gr-1+CD11b+F4/80+ cells were sorted from PBL of tumor-bearing mice. Fab fragments of the anti-NKG2D mAb or of an isotype-matched control mAb were added to cocultures of NK cells and Gr-1+CD11b+F4/80+ cells. Data are shown as mean plus or minus SD of triplicates. One representative experiment of 3 experiments is shown. *P < .05. ND indicates not detectable.
It was reported recently that the suppressive effect of MDSCs on T-cell proliferation is mediated by NO.14,15 To control for the activity of the NO inhibitor, we added L-NMMA or the inactive D-NMMA to the cocultures of Gr-1+CD11b+F4/80+ and splenocytes. In accordance with previous reports, the NO inhibitor L-NMMA, but not its inactive stereoisomeric isoform D-NMMA, completely reversed the inhibitory effect of MDSCs on T-cell proliferation (Figure 5B).
Using a transwell assay, we investigated whether cell-cell contact was required for IFN-γ production. Our data demonstrate that using a transwell the amounts of IFN-γ in cocultures of NK cells and Gr-1+CD11b+F4/80+ were significantly reduced, but not completely abrogated, indicating that IFN-γ production is partially dependent on cell-cell contact (Figure 5C). Because Gr-1+F4/80+ cells from blood of tumor-bearing mice express RAE-1 (Figure 1C), a ligand for the activating receptor NKG2D expressed on NK cells, we added Fab fragments of a blocking antibody directed against NKG2D or of the respective isotype-matched control Fab to the cocultures. Addition of Fab fragments of anti-NKG2D mAb to the cocultures significantly reduced the secretion of IFN-γ, whereas Fab fragments of the isotype-matched control mAb had no effect (Figure 5D). Addition of mAbs directed against other NK-cell receptors, including 2B4 and DNAM-1, did not reduce the amounts of IFN-γ produced in the cocultures (data not shown). Taken together, our data demonstrate that induction of IFN-γ production by NK cells mediated by MDSCs from tumor-bearing mice is partially dependent on cell-cell contact and signaling via NKG2D.
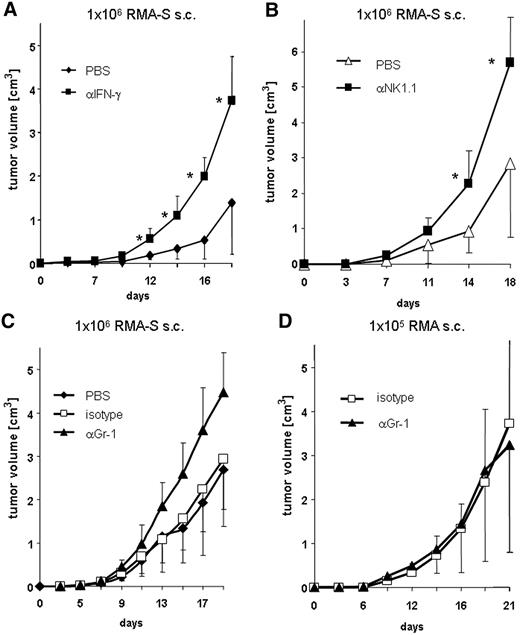
Neutralization of IFN-γ or the depletion of NK1.1+ or Gr-1+ cells leads to enhanced tumor growth of RMA-S lymphoma
We investigated the role of IFN-γ in the RMA-S tumor model. In concordance with previous reports,39 the neutralization of IFN-γ led to accelerated tumor growth (Figure 6A). In addition, depletion of NK cells led to faster tumor growth and the development of larger tumors, confirming that the growth of the RMA-S tumor was controlled by NK cells (Figure 6B). So far, tools are lacking to specifically deplete MDSCs from tumor-bearing mice. Several studies reported that depletion of the whole Gr-1+ cell compartment, which comprises MDSCs, from tumor-bearing mice improves the antitumor response mediated by CD8+ T cells and prevents and delays tumor growth.40,41 To investigate the role of MDSCs in the NK cell–sensitive RMA-S tumor model in vivo, Gr-1+ cells were depleted in mice inoculated with RMA-S tumor cells by using the mAb clone RB6-8C5. Of note, RMA-S cells do not express Gr-1 (data not shown). Figure 6C demonstrates that tumor growth was similar between PBS and isotype-matched control mAb–treated mice, whereas in mice treated with the anti–Gr-1 mAb, significantly accelerated tumor growth was observed. In contrast, depletion of Gr-1+ cells had no effect on tumor growth of the NK cell–resistant lymphoma RMA (Figure 6D). In conclusion, these data indicate that the presence of Gr-1+ cells leads to tumor control and slower growth of the NK cell–sensitive tumor RMA-S.
Neutralization of IFN-γ, and depletion of NK1.1+ or Gr-1+ cells in vivo leads to enhanced tumor growth. C57BL/6 mice (n = 10) were treated with PBS ( ), isotype-matched control mAb (□), a neutralizing anti–IFN-γ mAb (A, ■), a depleting anti-NK1.1 mAb (B, ■), or an anti–Gr-1 mAb (C and D, ▴) and were inoculated subcutaneously with either RMA-S (A,B,C) or RMA lymphoma cells (D). (A-D) Tumor growth was monitored and is presented as mean plus or minus SD. Asterisks indicate statistical significance of P < .01, determined by a one-way analysis of variance (ANOVA) test. One representative experiment of at least 2 independent experiments is shown.
), isotype-matched control mAb (□), a neutralizing anti–IFN-γ mAb (A, ■), a depleting anti-NK1.1 mAb (B, ■), or an anti–Gr-1 mAb (C and D, ▴) and were inoculated subcutaneously with either RMA-S (A,B,C) or RMA lymphoma cells (D). (A-D) Tumor growth was monitored and is presented as mean plus or minus SD. Asterisks indicate statistical significance of P < .01, determined by a one-way analysis of variance (ANOVA) test. One representative experiment of at least 2 independent experiments is shown.
Neutralization of IFN-γ, and depletion of NK1.1+ or Gr-1+ cells in vivo leads to enhanced tumor growth. C57BL/6 mice (n = 10) were treated with PBS ( ), isotype-matched control mAb (□), a neutralizing anti–IFN-γ mAb (A, ■), a depleting anti-NK1.1 mAb (B, ■), or an anti–Gr-1 mAb (C and D, ▴) and were inoculated subcutaneously with either RMA-S (A,B,C) or RMA lymphoma cells (D). (A-D) Tumor growth was monitored and is presented as mean plus or minus SD. Asterisks indicate statistical significance of P < .01, determined by a one-way analysis of variance (ANOVA) test. One representative experiment of at least 2 independent experiments is shown.
), isotype-matched control mAb (□), a neutralizing anti–IFN-γ mAb (A, ■), a depleting anti-NK1.1 mAb (B, ■), or an anti–Gr-1 mAb (C and D, ▴) and were inoculated subcutaneously with either RMA-S (A,B,C) or RMA lymphoma cells (D). (A-D) Tumor growth was monitored and is presented as mean plus or minus SD. Asterisks indicate statistical significance of P < .01, determined by a one-way analysis of variance (ANOVA) test. One representative experiment of at least 2 independent experiments is shown.
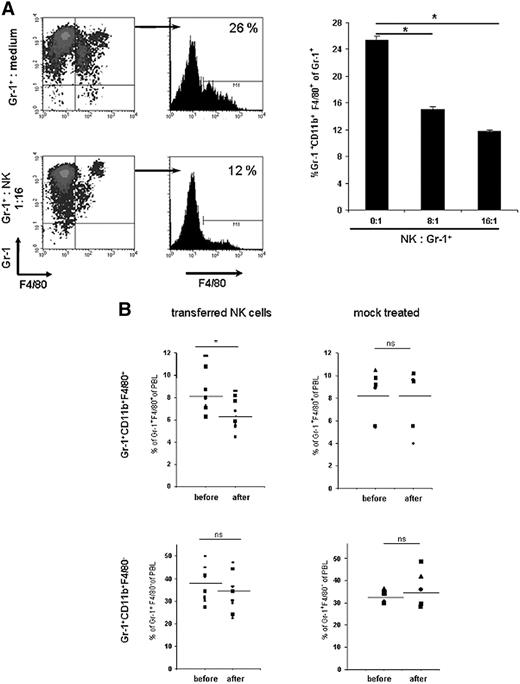
Gr-1+CD11b+F4/80+ cells are eliminated by activated NK cells
RAE-1–expressing targets are efficiently killed by IL-2–activated NK cells.19,20 Therefore, we examined whether Gr-1+CD11b+F4/80+RAE-1+ cells were eliminated by activated NK cells. Gr-1+ cells from blood of tumor-bearing mice were incubated at different effector-target ratios with IL-2–activated NK cells and the percentages of Gr-1+CD11b+F4/80+ of total Gr-1+ cells were determined by flow cytometry. In the analysis, NK cells were excluded by gating on NK1.1-negative cells. Importantly, highly reduced percentages of Gr-1+CD11b+F4/80+ cells among Gr-1+ cells were detected in cocultures with NK cells as compared with cultures in medium alone, and this reduction depended on the effector-to-target ratio (Figure 7A). Addition of an anti-NKG2D mAb that blocks NKG2D and RAE-1 interactions in cytotoxic assays42 failed to prevent NK cell–mediated elimination of MDSCs (data not shown). Of note, numbers of Gr-1−F4/80+ did not significantly increase upon coculture with NK cells (Figure 7A). We also did not detect higher numbers of mature myeloid cells, such as CD11c+ DCs, after coculture of MDSCs with NK cells compared with MDSCs cultured alone (data not shown).
Gr-1+CD11b+F4/80+ cells are eliminated by NK cells in vitro and in vivo. (A) IL-2–activated NK cells were cultured with Gr-1+ cells isolated from PBLs of tumor-bearing mice at the indicated effector-target ratios for 12 hours. Percentages of Gr-1+CD11b+F4/80+ cells among Gr-1+ were determined by flow cytometry. Data are presented as mean plus or minus SD of triplicate cultures. One representative experiment of 3 independent experiments is shown. (B) NK cells were isolated from naive C57BL/6 mice. NK cells (4-6 × 106) were injected intravenously into C57BL/6-Ly5.1 tumor-bearing mice (n = 9) at day 18 or 19 after tumor cell inoculation. Percentages of Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− cells among total PBLs were determined by flow cytometry 4 hours before and 5 hours after transfer. Injected NK cells were excluded by gating on Ly5.2-negative cells. In control experiments, mice were mock-treated as described in “Methods.” *P < .05. NS indicates not statistically significant.
Gr-1+CD11b+F4/80+ cells are eliminated by NK cells in vitro and in vivo. (A) IL-2–activated NK cells were cultured with Gr-1+ cells isolated from PBLs of tumor-bearing mice at the indicated effector-target ratios for 12 hours. Percentages of Gr-1+CD11b+F4/80+ cells among Gr-1+ were determined by flow cytometry. Data are presented as mean plus or minus SD of triplicate cultures. One representative experiment of 3 independent experiments is shown. (B) NK cells were isolated from naive C57BL/6 mice. NK cells (4-6 × 106) were injected intravenously into C57BL/6-Ly5.1 tumor-bearing mice (n = 9) at day 18 or 19 after tumor cell inoculation. Percentages of Gr-1+CD11b+F4/80+ and Gr-1+CD11b+F4/80− cells among total PBLs were determined by flow cytometry 4 hours before and 5 hours after transfer. Injected NK cells were excluded by gating on Ly5.2-negative cells. In control experiments, mice were mock-treated as described in “Methods.” *P < .05. NS indicates not statistically significant.
Adoptive transfer of NK cells reduces numbers of Gr-1+CD11b+F4/80+ cells in tumor-bearing mice
In order to investigate whether NK cells eliminate Gr-1+CD11b+F4/80+ cells in tumor-bearing mice in vivo, NK cells isolated from naive mice were injected intravenously into tumor-bearing mice at day 18 or 19 after tumor cell inoculation. The percentages of Gr-1+CD11b+F4/80+ cells among PBLs 4 hours before and 5 hours after injection of NK cells were determined by flow cytometry. Figure 7B illustrates that a significant reduction of Gr-1+CD11b+F4/80+ cells was detectable after adoptive transfer of NK cells, but not in mock-treated animals. The percentages of Gr-1+CD11b+F4/80− cells were not significantly altered. We conclude that Gr-1+CD11b+F4/80+ cells in RMA-S tumor-bearing mice are also eliminated in vivo by adoptively transferred NK cells.
Discussion
It is well established that MDSCs accumulate in cancer patients and in tumor-bearing mice and suppress antitumor T-cell responses. Therefore, substantial effort is dedicated to the discovery of novel strategies to destroy MDSCs and/or eliminate their function, in order to enhance T-cell activation against cancer. Our study investigated whether MDSCs also regulate NK cell–mediated antitumor immune responses against the TAP2-negative and MHC class I–deficient RMA-S lymphoma. Unexpectedly, we discovered that Gr-1+CD11b+F4/80+ MDSCs, which suppressed T-cell proliferation, potently activated NK cells to produce high amounts of IFN-γ. These results suggest that under certain circumstances strategies aimed at the elimination of MDSCs might not be beneficial to induce successful immune responses against NK cell–sensitive tumors.
Several recent studies correlated phenotypical features of MDSCs with their suppressive function on T cells. Our data confirmed that 2 subpopulations within Gr-1+CD11b+ MDSCs can be distinguished by differential expression of Ly6G and a distinct forward and side scatter profile. The subset of mononuclear MDSCs (MO-MDSCs) expresses the monocyte/macrophage marker F4/80. In concordance with previous reports,8 we observed that the Gr-1+CD11b+F4/80+ population, which accumulated in RMA-S tumor-bearing mice, expressed higher levels of IL-4Rα (CD124), as compared with naive mice, and expressed high levels of CD115 (data not shown). Importantly, we discovered that the NKG2D ligand RAE-1 was exclusively expressed on Gr-1+CD11b+F4/80+ MDSCs from RMA-S, RMA-RAE-1γ lymphoma, and B16BL6 melanoma tumor-bearing mice. RAE-1 expression was not detectable on PBLs from healthy mice. The exact mechanisms leading to the up-regulation of RAE-1 on MDSCs are currently unknown.
A bidirectional cross-talk exists between NK cells and myeloid cells, including immature dendritic cells, mature dendritic cells, and macrophages isolated from healthy individuals or mice.27,43 The goal of our study was to determine the interaction of NK cells with MDSCs from tumor-bearing mice. In our study, NK-cell proliferation and cytotoxic activity were not affected upon coculture with Gr-1+CD11b+F4/80+ cells or Gr-1+CD11b+F4/80− cells isolated from tumor-bearing mice. In this context, Liu et al31 reported recently that NK cell–mediated killing of YAC-1 cells was inhibited after a 5-day coculture of Gr-1+CD11b+ cells in presence of IL-2. Upon coculture with MDSCs in the presence of IL-2, NK cells expressed decreased levels of STAT5, suggesting that MDSCs interfered with IL-2 signaling. In our experiments, we did not observe an effect of MDSCs on the cytolytic activity of freshly isolated NK cells without any further activation (eg, by the exogenous addition of cytokines). Collectively, these results imply that MDSCs may differentially interfere with signaling pathways during NK-cell activation.
Unexpectedly, we discovered that MDSCs from tumor-bearing mice induced high amounts of IFN-γ production by NK cells in the absence and in the presence of IL-12. The Gr-1+CD11b+F4/80+ MDSC population was a much more potent inducer of IFN-γ as compared with the Gr-1+CD11b+F4/80− subpopulation. Several studies demonstrated that immature and mature DCs isolated from healthy individuals induce IFN-γ production by resting NK cells.25,44 In our study, Gr-1+CD11b+F4/80+ cells isolated from healthy mice induced lower amounts of IFN-γ, as compared with cells isolated from tumor-bearing mice (data not shown). Therefore, we assume that Gr-1+CD11b+F4/80+ cells isolated from tumor-bearing mice differ not only quantitatively, but also qualitatively, from Gr-1+CD11b+F4/80+ cells derived from healthy mice. Indeed, our data demonstrate that Gr-1+CD11b+F4/80+ cells from tumor-bearing mice, but not from healthy mice, expressed the NKG2D ligand RAE-1. Since the abrogation of IFN-γ production upon addition of the blocking mAb directed against NKG2D was only partial, we assume that additional mechanisms, most likely mediated by soluble factors, are involved.
The antitumor activity of IFN-γ is well documented in different tumor models, including the RMA-S lymphoma.39 Our data confirm that in the RMA-S tumor model neutralization of IFN-γ leads to significantly accelerated tumor growth. Similar results were obtained in Rag-2−/− mice, suggesting that IFN-γ controls tumor growth in the absence of T cells. IFN-γ does not directly affect the proliferation of RMA-S cells in vitro (data not shown). The exact role of IFN-γ in the innate antitumor immune response against RMA-S tumors is still elusive. Of note, we have evidence that IFN-γ mediates the accumulation of NK cells in the tumors (I.E.G., unpublished observation). Therefore, it is possible that enhanced levels of IFN-γ, as observed in the NK-cell and MDSC cocultures, might cause the accumulation of higher numbers of cytolytic NK cells in the tumors that control tumor growth.
Immature DCs isolated from healthy individuals25,45 or LPS-stimulated macrophages30 are eliminated by activated NK cells. In our study, Gr-1+CD11b+F4/80+ MDSCs also were eliminated during coculture with NK cells. The disappearance of Gr-1+CD11b+F4/80+ cells after coculture with NK cells could either be due to NK cell–mediated killing or to an induction of cell differentiation with a change of phenotype. In these cocultures we did not observe a substantial increase in cells carrying markers of differentiated myeloid cells, including CD11c+ DCs (data not shown), implying that the induction of myeloid cell differentiation by NK cells might play a subordinate role in our model. Since the reduction of MDSCs in vivo was already observed 5 hours after NK-cell adoptive transfer, we assume that the reduction was due to a direct cytolytic effect of NK cells on MDSCs. We believe that within 5 hours after transfer of NK cells a reduction of MDSCs due to a reduction of tumor mass is unlikely. The elimination of Gr-1+CD11b+F4/80+ cells was only minimally affected by the addition of a mAb directed against NKG2D (data not shown), indicating that other receptors or soluble factors are involved.
Importantly, impaired NK-cell activity has been described in cancer patients.46-48 In tumor-bearing mice at the time when MDSCs start to expand, some NK cells might already be inactivated by the tumor. To circumvent a possible tumor-induced inactivation of NK cells in tumor-bearing hosts, we adoptively transferred freshly isolated syngeneic NK cells into tumor-bearing hosts and monitored MDSC numbers in the blood. After adoptive transfer of NK cells, a significant decrease in Gr-1+CD11b+F4/80+ MDSCs, but not Gr-1+CD11b+F4/80− cells, was observed. Adoptive immunotherapy of NK cells into cancer patients is currently being optimized in clinical trials (reviewed in Ljunggren and Malmberg49 ). Our study suggests a dual benefit from NK-cell adoptive immunotherapy against cancer. First, NK cells directly attack and eliminate tumor cells. Second, NK cells have the potential to facilitate T-cell activation and priming of efficient Th1 responses to the tumor by the production of high amounts of IFN-γ and by eliminating MDSCs to restore T-cell effector functions.
So far, tools to specifically eliminate MDSCs are lacking. Depletion of the whole Gr-1+ compartment, which includes MDSCs, is commonly used to understand the biologic function of MDSCs.35,40,41 We observed that in the NK cell–dependent RMA-S tumor model, but not in the NK cell–resistant RMA model, depletion of Gr-1+ cells accelerated tumor growth. Our data show that elimination of Gr-1+ cells comprising MDSCs in a NK cell–dependent tumor model does not improve, but rather impairs, antitumor immune responses. In contrast, in order to induce optimal tumor-specific T-cell responses (eg, by dendritic cell vaccination protocols), it is of major importance to block the function of MDSCs. Our data suggest that it might be advantageous to block factors released by MDSCs that mediate T-cell suppression, such as peroxynitrite,12 rather than to eliminate MDSCs, to optimally enhance immune responses against cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sascha Hinterkopf and Joachim Neuert for excellent technical assistance; J.P. Houchins (R&D Systems) for providing mAbs directed against RAE-1; Prof A. Shibuya for providing the anti-DNAM mAb; Dr Kai Zanzinger, Dr Carola Schellack, Prof Peter Angel, Dr Marina Schorpp-Kistner, and Björn Textor for helpful discussions; Prof Lewis Lanier, Dr Natalio Garbi, and Dr Elisabeth Suri-Payer for critically reading the manuscript.
This work was supported by Boehringer Ingelheim Fonds, Deutsche José Carreras Leukämie Stiftung, and Marie Curie Excellence Grant (A.C.).
Authorship
Contribution: N.N. conducted experiments and wrote the manuscript; I.E.G. and E.S. conducted experiments; and A.C. planned and supervised experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adelheid Cerwenka, Division of Innate Immunity, German Cancer Research Center, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: a.cerwenka@dkfz.de.