Abstract
Cytomegalovirus (CMV) and its therapy continue to contribute to morbidity and mortality in hemopoietic stem cell transplantation (HSCT). Many studies have demonstrated the feasibility of in vitro generation of CMV-specific T cells for adoptive immunotherapy of CMV. Few clinical trials have been performed showing the safety and efficacy of this approach in vivo. In this study, donor-derived, CMV-specific T cells were generated for 12 adult HSCT patients by stimulation with dendritic cells transduced with an adenoviral vector encoding the CMV-pp65 protein. Patients received a prophylactic infusion of T cells after day 28 after HSCT. There were no infusion related adverse events. CMV DNAemia was detected in 4 patients after infusion but was of low level. No patient required CMV-specific pharmacotherapy. Immune reconstitution to CMV was demonstrated by enzyme linked immunospot assay in all recipients with rapid increases in predominantly CMV-pp65 directed immunity in 5. Rates of graft-versus-host disease, infection, and death were not increased compared with expected. These results add to the growing evidence of the safety and efficacy of immunotherapy of CMV in HSCT, supporting its more widespread use. This study was registered at www.anzctr.org.au as #ACTRN12605000213640.
Introduction
Although effective cytomegalovirus (CMV)–specific pharmacotherapy exists, CMV remains a significant problem after hemopoietic stem cell transplantation (HSCT). Ultimately, only CMV-specific immune reconstitution adequately controls CMV reactivation and disease. Adoptive transfer of CMV-specific T cells has the potential to safely restore CMV-specific immunity, control active CMV, and prevent CMV recurrence. Although there are a plethora of in vitro studies demonstrating the feasibility of producing CMV-specific T cells for adoptive transfer, the number of clinical trials using such cells remains small.1-7 Ongoing trials are needed to establish safety and efficacy and optimize clinically successful techniques. Studies examining adoptive immunotherapy of CMV have varied in their source of antigen used to generate the CMV-specific T cells.
We have previously shown the safety of using the HLA-A2 restricted epitope of the pp65 antigen (NLVPMVATV) to generate a highly specific population of predominantly CD8+ cytotoxic T cells. These cells were given to 9 patients (age range, 4-65 years), the majority receiving a T cell–replete graft after nonmyeloablative conditioning. Infusion was not associated with toxicity, and there was no increase in graft-versus-host disease (GVHD) compared with expected rates. CMV reactivation as measured by CMV polymerase chain reaction (PCR) occurred in 2 patients, neither of whom required specific anti-CMV therapy. CMV-specific immunity as measured by tetramer analysis was seen to rise transiently in 6 of these patients.8
There are 2 significant limitations inherent to this technique: (1) the HLA restriction of a single epitope makes its broad application to all patients undergoing HSCT difficult; and (2) although being highly specific for CMV, the infusional product lacks specific CD4+ helper T cells, which has been suggested to be important for maintenance of functional T-cell immunity.9,10 These limitations have not been an issue with earlier immunotherapy studies using live virus1,2 or inactivated viral lysates.3,4 However, using these antigens does not conform to good manufacturing practice guidelines, making their inclusion in immunotherapy protocols untenable.
Recently, the Houston group used an adenoviral vector encoding the immunodominant pp65 lower matrix protein antigen (Ad5f35pp65) to simulate CMV-specific T cells. Combined with Epstein-Barr virus (EBV)–transformed B cells as antigen-presenting cells, they generated cultures with activity against CMV, adenovirus, and EBV. These cells were then given prophylactically to 11 patients, most of whom had undergone in vivo T-cell depletion.5
Given the inherent limitations of using a single epitope to stimulate CMV-specific T cells, we proceeded to use the Ad5f35pp65 vector to generate CD4+ and CD8+ CMV-specific T cells for adoptive transfer in HSCT. Here we describe a phase 1 clinical trial examining the safety and efficacy of this technique in 12 mostly non–T cell–depleted patients infused prophylactically after allogeneic HSCT, confirming the utility of this technique in a broader range of patients.
Methods
Participant details
Patients of all HLA types receiving an allogeneic HSCT for hematologic malignancy from a fully matched or one antigen-mismatched related or unrelated donor were eligible for inclusion in this study. Patients with a life expectancy of less than 6 months and with CMV-seronegative donors were excluded. This study was approved by the institutional ethics committee, and informed consent was obtained from the donor and recipient before enrollment in accordance with the Declaration of Helsinki. Patients received CMV-specific T-cell infusions between January 8, 2007, and November 5, 2007. This research was approved by the Human Research Ethics committees of Westmead Hospital and the University of Sydney. In addition, protocols received approval from the Gene and related Therapies Research Advisory Panel of the National Health and Medical Research Council.
T-cell generation
CMV-pp65-specific T cells were generated as previously described.11 Briefly, dendritic cells (DCs) were differentiated from donor monocytes isolated from peripheral blood mononuclear cells (PBMCs) by adherence. These were cultured for 7 days in Cellgro serum-free medium (Cellgenix, Freiburg, Germany) supplemented with recombinant human interleukin-4 and granulocyte-macrophage colony-stimulating factor 1000 U/mL (Chemicon International, Temecula, CA). DCs were matured on day 6 by the addition of recombinant human tumor necrosis factor-α (Chemicon International). The Ad5f35pp65 vector was added to the culture simultaneously at a multiplicity of infection of 20 (supplied by the Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX). The multiplicity of infection was based on preclinical experiments studying NLV+ tetramer cell expansion in HLA-A2+ CMV-seropositive persons after Ad5f35pp65 transduction of DC. Mature DCs were harvested, washed, irradiated 30 Gy, suspended in AIM-V (Invitrogen, Carlsbad, CA) with 10% human AB serum (ARCBS, Sydney, Australia), and used to stimulate autologous PBMCs at an effector-to-stimulator ratio of 10:1. T-cell cultures were restimulated on day 7 at which time recombinant human IL-2 (Chemicon International) was added at 20 U/mL. The concentration of IL-2 was increased to 50 U/mL on day 14, and cells were harvested and cryopreserved on day 21 for later analysis and infusion.
T-cell infusion
T cells were infused at 2 × 107/m2 on or after day 28 after HSCT. This dose was chosen based on prior data from the Houston group regarding prophylaxis of EBV infection, the number of non–CMV-specific cells in our products, and the risk of GVHD with nonspecific donor lymphocyte infusions.12,13 The primary endpoint was infusional safety with secondary endpoints of CMV reactivation, incidence of GVHD, use of antivirals, and CMV-specific immune reconstitution.
Infusion was delayed if there was grade 2 or greater GVHD, corticosteroid dose equivalent to or greater than 1 mg/kg per day prednisolone, antilymphocyte globulin use within the 4 weeks before intended infusion, and significant renal or liver dysfunction (creatinine or bilirubin greater than twice the upper limit of normal range, serum transaminases greater than 3 times the upper limit of normal range).
Release criteria for cell products included negative cultures for bacteria and fungi, negative testing for Mycoplasma by PCR, greater than 50% after thaw recovery and viability by trypan blue exclusion, less than 2% contaminating CD19+ B cells and CD14+ monocytes as analyzed by flow cytometry, and negative alloreactivity (< 10% killing of recipient phytohemagglutinin (PHA) blasts at an effector-to-target ratio of 20:1 assessed by 4-hour 51Cr release assay).
Postinfusion immunophenotyping and tetramer staining
Immune reconstitution directed against individual CMV-pp65 epitopes after infusion was monitored by immunophenotype and tetramer binding. All persons infused had at least one HLA type corresponding to one of 5 specific epitopes for which tetramers were commercially available (Beckman Coulter, Fullerton, CA). These included HLA A*0201 (NLVPMVATV), HLA A*2402 (QYDPVAALF), HLA B*0702 (TPRVTGGGAM), HLA B*0801 (ELRRKMMYM), and HLA B*3501 (IPSINVHHY). Antibodies directed against CD3, CD4, CD8, CD19, CD56, and CD14 were all purchased from BD Biosciences (San Jose, CA).
Tetramer was added to 100 μL of whole blood and incubated at room temperature for 30 minutes. Antibodies for cell surface antigens were added for the final 15 minutes of incubation followed by lysis of red cells using PharmLyse (BD Biosciences). Cells were then washed and resuspended in flow buffer consisting of phosphate-buffered saline/1% bovine serum albumin. A minimum of 30 000 events was acquired on the LSRII flow cytometer with FACSDiva software (BD Biosciences). Results were analyzed using FCS Express (De Novo Software, Los Angeles, CA).
Absolute tetramer+ cell numbers present in the peripheral blood were calculated by multiplying the percentage of tetramer+ cells by the white cell count obtained from the Advia full blood count analyzer.
Postinfusion enzyme-linked immunospot assay
Reconstitution to whole pp65, IE1, and adenoviral proteins was assessed by interferon-γ enzyme linked immunospot assay (ELISPOT) analysis as previously described.8 A total of 0.5 to 1 × 105 cells from each time point were suspended in 200 μL AIM-V/10% human AB serum and stimulated with pp65, IE1, and adenoviral hexon protein pepmix (JPT Peptide Technologies, Berlin, Germany) for 18 hours in multiscreen, MAIPS4510 96-well filter plates (Millipore, Billerica, MA) precoated with catcher antibody (m-AB 1-DIK) (BD Biosciences). After washing and incubation with detector antibody (m-AB 7-B6-1-Biotin) (BD Biosciences), spots were developed using ExtrAvidin and SigmaFast BCIP/NBT alkaline phosphatase substrate (Sigma-Aldrich, St Louis, MO) according to the manufacturer's directions. Spots were counted manually and results expressed as spot-forming cells per 105 cells. Testing was performed in triplicate for each time point. Preinfusion and postinfusion samples were batched to avoid interassay variability.
CMV surveillance and therapy
Monitoring for CMV reactivation consisted of weekly qualitative PCR. If positive, a quantitative PCR was then performed. Ganciclovir therapy 5 mg/kg twice daily was initiated by an independent treating physician if CMV DNA levels exceeded 1500 copies/mL. Definitions of CMV reactivation, infection, and disease are as per Ljungman et al.14
Monitoring for adverse events
Patients were monitored for infusional toxicity, infection, and GVHD. To assess infusional toxicity, patients were monitored for 4 hours and reviewed 24 hours after T-cell infusion. GVHD was graded according to Glucksberg et al.15
Results
Safety of CMV-specific T-cell infusion
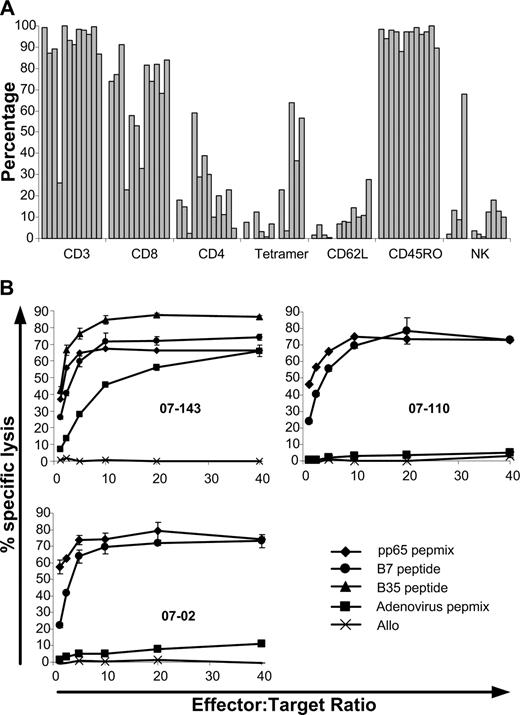
Between January and December of 2007, 12 patients received a single dose of 2 × 107/m2 CMV-specific T cells. Patient age ranged from 18 to 65 years; 6 received myeloablative conditioning and 4 received in vivo T-cell depletion (2 with alemtuzumab and 2 with antithymocyte globulin; Table 1). In all cases, in vitro cultures produced adequate numbers for the target cell dose. With a median starting number of PBMCs of 14 × 106 (range, 8 × 106 - 20 × 106), cultures expanded almost 20-fold (median, 19.5-fold; range, 8.5-fold to 30-fold) over a 21-day period to a median of 211 × 106 (range, 136 × 106 - 505 × 106) total cells. Cells infused were predominantly CD3+ (median, 94.5%; range, 26%-99.8%), with a greater proportion of CD8+ (median, 73.9%; range, 23%-91%) compared with CD4+ (median, 19%; range, 2.5%-59%) T cells. Single CMV epitope restriction based on tetramer binding ranged from 0.2% to 64% (median, 7.7%). This varied depending on the individual epitope. Cultures from 4 HLA A*0201+ donors had a higher proportion of tetramer+ cells (median, 32.2%; range, 3.3%-64%) than the 4 HLA B*0702+ donors (median, 17.6%; range, 6.9%-36.5%) or the 3 HLA B*3501 (median, 0.4%; range, 0.2%-3.8%). Culture from 1 HLA A*2401 donor had 0.7% tetramer+ cells. Tetramer+ binding for the single culture from an HLA B*0801+ donor was 0.05%. In 2 cultures, specificity for the HLA A*0101-restricted epitope of the adenoviral hexon protein (TDLGQNLLY) was assessed by pentamer staining (ProImmune, Oxford, United Kingdom). In these cultures, 5.3% and 15% of the cells were specific for this epitope. All cultures lysed pp65 pulsed target cells (median specific lysis, 65%; range, 42.6%-82.5%) with lower lysis of adenovirus hexon protein-pulsed targets (median specific lysis, 4.8%; range, 0%-71%). All cultures had less than 5% lysis of unpulsed allogeneic target cells (median specific lysis, 0.1%; range, 0%-3.6%). Phenotypic and functional characteristics of the T-cell products infused can be seen in Figure 1. Patient characteristics can be found in Table 1. At the time of infusion, all patients were receiving prophylactic cyclosporin. Five patients were taking oral prednisolone at a dose of less than 1 mg/kg per day (patient 0702, 0.25 mg/kg per day for gout; patient 06205, 0.5 mg/kg per day for prior GVHD; patient 0775, 0.3 mg/kg per day for prior GVHD; patient 07110, 0.6 mg/kg per day for red cell aplasia after transplant; patient 07138, 0.25 mg/kg per day for prior GVHD). All patients except for 2 were receiving standard prophylactic oral dose valaciclovir of 500 mg daily. Patient 07110 was taking oral valaciclovir 2 g 4 times per day for genital warts and patient 07143 was receiving intravenous foscarnet 6 g 2 times per day for multiple prior episodes of CMV reactivation. This was ceased 2 days after T-cell infusion.
Patient characteristics
| Patient no. . | Age, y . | Sex . | Diagnosis . | Disease status . | Conditioning . | MUD vs MSD . | CD34+ dose (×106/kg) . | Day to engraftmen‡ . | CMV status . | Immunosuppression . | Antivirals§ . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 06213 | 35 | F | AML | CR1 | Flu/Mel | MSD | 8.5 | 13 | Positive | CSA | — |
| 0702 | 56 | M | MDS/AML | PR | Flu/Mel/alemtuzumab† | MUD | 8.7 | 24 | Positive | CSA/Pred | — |
| 06205 | 51 | F | ALL | CR1 | Cy/TBI | MSD | 2.6 | 26 | Positive | CSA/Pred | — |
| 0735 | 19 | F | ALL | CR2 | Cy/MESNA/TBI | MSD | 4.4 | 17 | Negative | CSA | — |
| 0743 | 65 | M | CML | CP2* | Flu/Mel | MSD | 3.7 | 27 | Positive | CSA | — |
| 0775 | 54 | M | AML | CR2 | Flu/Mel/ATG† | MUD | 6.9 | 17 | Negative | CSA/Pred | — |
| 0786 | 19 | M | AML | CR2 | Bu/Cy | MSD | 6 | 18 | Positive | CSA | — |
| 07110 | 39 | M | AML | CR1 | Bu/Cy | MSD | 3.3 | 26 | Positive | CSA/Pred | Val |
| 07141 | 49 | M | AML | CR1 | Bu/Cy | MSD | 2.1 | 14 | Negative | CSA | — |
| 07143 | 18 | M | AML | CR1 | Flu/Mel/alemtuzumab† | MUD | 4.6 | 14 | Positive | CSA | Foscarnet |
| 07138 | 39 | F | AML | CR1 | Cy/TBI/ATG† | MUD | 2.1 | 22 | Positive | CSA/Pred | — |
| 07169 | 51 | M | AML | CR1* | Flu/Mel | MSD | 4.4 | 30 | Positive | CSA | — |
| Patient no. . | Age, y . | Sex . | Diagnosis . | Disease status . | Conditioning . | MUD vs MSD . | CD34+ dose (×106/kg) . | Day to engraftmen‡ . | CMV status . | Immunosuppression . | Antivirals§ . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 06213 | 35 | F | AML | CR1 | Flu/Mel | MSD | 8.5 | 13 | Positive | CSA | — |
| 0702 | 56 | M | MDS/AML | PR | Flu/Mel/alemtuzumab† | MUD | 8.7 | 24 | Positive | CSA/Pred | — |
| 06205 | 51 | F | ALL | CR1 | Cy/TBI | MSD | 2.6 | 26 | Positive | CSA/Pred | — |
| 0735 | 19 | F | ALL | CR2 | Cy/MESNA/TBI | MSD | 4.4 | 17 | Negative | CSA | — |
| 0743 | 65 | M | CML | CP2* | Flu/Mel | MSD | 3.7 | 27 | Positive | CSA | — |
| 0775 | 54 | M | AML | CR2 | Flu/Mel/ATG† | MUD | 6.9 | 17 | Negative | CSA/Pred | — |
| 0786 | 19 | M | AML | CR2 | Bu/Cy | MSD | 6 | 18 | Positive | CSA | — |
| 07110 | 39 | M | AML | CR1 | Bu/Cy | MSD | 3.3 | 26 | Positive | CSA/Pred | Val |
| 07141 | 49 | M | AML | CR1 | Bu/Cy | MSD | 2.1 | 14 | Negative | CSA | — |
| 07143 | 18 | M | AML | CR1 | Flu/Mel/alemtuzumab† | MUD | 4.6 | 14 | Positive | CSA | Foscarnet |
| 07138 | 39 | F | AML | CR1 | Cy/TBI/ATG† | MUD | 2.1 | 22 | Positive | CSA/Pred | — |
| 07169 | 51 | M | AML | CR1* | Flu/Mel | MSD | 4.4 | 30 | Positive | CSA | — |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; Bu, busulphan; CML, chronic myeloid leukemia; CP2, second chronic phase; CR, complete remission; CSA, cyclosporine A; Cy, cyclophosphamide; Flu, fludarabine; MDS, myelodysplastic syndrome; Mel, melphalan; MSD, matched sibling donor; MUD, matched unrelated donor; PR, partial remission; Pred, prednisolone; TBI, total body irradiation; and Val, valaciclovir.
Second transplantation after prior allogeneic transplantation for CML.
In vivo T-cell depletion included antithymocyte globulin or alemtuzumab as part of the conditioning regimen in 4 patients.
The days to neutrophil engraftment, defined as a peripheral blood neutrophil count greater than 109/L on 2 or more successive days.
Therapeutic antivirals, excluding standard prophylactic valaciclovir 500 mg/day given from day 1 after HSCT until immune reconstitution. All patients were receiving prophylactic valaciclovir at the time of infusion except patients 07110 and 07143 who were receiving valaciclovir 2 g four times per day and therapeutic foscarnet, respectively.
Phenotypic and functional characteristics of the T-cell products infused. (A) Phenotypic characteristics of T cells infused. CD3+, CD56+, and Tetramer+ cells are expressed as a percentage of total cells. CD8+, CD4+, CD62L+, and CD45RO+ cells are expressed as a percentage of CD3+ cells. (B) Function of T cells infused as assessed by chromium release assay. (Top left plot) An example of killing of recipient PHA blasts pulsed with CMV-pp65 pepmix (♦), HLA-B*0702 restricted (•), or HLA-B*3501 (▴) restricted CMV peptides as well as adenoviral hexon protein pepmix (■). Top right and bottom left plots show examples of killing of recipient PHA blasts pulsed with CMV-pp65 pepmix or HLA-B7–restricted CMV-peptide with more typical low-level killing of blasts pulsed with adenoviral hexon protein pepmix. In all 3 plots, typical absence of killing of unpulsed allo-PHA blasts (×) is seen.
Phenotypic and functional characteristics of the T-cell products infused. (A) Phenotypic characteristics of T cells infused. CD3+, CD56+, and Tetramer+ cells are expressed as a percentage of total cells. CD8+, CD4+, CD62L+, and CD45RO+ cells are expressed as a percentage of CD3+ cells. (B) Function of T cells infused as assessed by chromium release assay. (Top left plot) An example of killing of recipient PHA blasts pulsed with CMV-pp65 pepmix (♦), HLA-B*0702 restricted (•), or HLA-B*3501 (▴) restricted CMV peptides as well as adenoviral hexon protein pepmix (■). Top right and bottom left plots show examples of killing of recipient PHA blasts pulsed with CMV-pp65 pepmix or HLA-B7–restricted CMV-peptide with more typical low-level killing of blasts pulsed with adenoviral hexon protein pepmix. In all 3 plots, typical absence of killing of unpulsed allo-PHA blasts (×) is seen.
T cells were given at a median of 55 days (range, 31-101 days) after HSCT. Reason for delay included 3 with GVHD (patients 06205, 0775, 07138), 1 resulting from corticosteroids for red cell aplasia (07110), 2 with transiently elevated creatinine (06213, 07169), 1 with intractable nausea and vomiting (0786), 1 with persistent fevers (07143), 3 resulting from cell product or patient availability (0702, 0735, 0743, all less than 2 weeks), and 1 resulting from patient preference (07141). There were no adverse reactions to the infusion within the first 24 hours; however, 1 recipient (0743) had a self-limiting episode of dizziness and weakness 24 hours after infusion, not associated with any evidence of focal neurologic deficits, hemodynamic changes, or sepsis. This was consistent with multiple prior episodes and was not thought to be related to the T cells. A summary of the incidence of CMV reactivation, GVHD, infections, and other adverse events can be found in Table 2.
Postinfusion progress of patients receiving CMV-specific T cells
| Patient no. . | Day of infusion . | Dose,107/m2 . | Infusional adverse events, Y/N . | Last day of follow-up . | CMV reactivation* . | GVHD† . | Non-CMV infection‡Y/N . | Relapse . | Other§Y/N . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y/N . | Day . | Treatment . | Y/N . | Day . | Grade . | Y/N . | Day . | |||||||
| 06213 | 45 | 2 | N | 419 | Y | 46 146 | Nil | Y | 90, 201 | III | N | N | N | |
| 0702 | 31 | 2 | N | 239 | Y | 38 | Val | N | N | N | Y | |||
| 06205 | 101 | 2 | N | 453 | N | Y | 220 | II | Y | N | N | |||
| 0735 | 38 | 2 | N | 359 | N | N | N | Y | 174 | Y | ||||
| 0743 | 38 | 2 | N | 335 | Y | 45 | Nil | Y | 171 | II | N | N | Y | |
| 0775 | 59 | 2 | N | 311 | N | N | N | Y | 166 | N | ||||
| 0786 | 61 | 2 | N | 288 | N | N | N | N | N | |||||
| 07110 | 68 | 2 | N | 239 | N | N | N | N | N | |||||
| 07141 | 50 | 2 | N | 201 | N | N | N | N | N | |||||
| 07143 | 45 | 2 | N | 214 | Y | 50 101 | Val | Y | 87 | II | Y | N | N | |
| 07138 | 73 | 2 | N | 213 | N | N | N | Y | 137 | N | ||||
| 07169 | 61 | 2 | N | 155 | N | N | N | N | N | |||||
| Patient no. . | Day of infusion . | Dose,107/m2 . | Infusional adverse events, Y/N . | Last day of follow-up . | CMV reactivation* . | GVHD† . | Non-CMV infection‡Y/N . | Relapse . | Other§Y/N . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y/N . | Day . | Treatment . | Y/N . | Day . | Grade . | Y/N . | Day . | |||||||
| 06213 | 45 | 2 | N | 419 | Y | 46 146 | Nil | Y | 90, 201 | III | N | N | N | |
| 0702 | 31 | 2 | N | 239 | Y | 38 | Val | N | N | N | Y | |||
| 06205 | 101 | 2 | N | 453 | N | Y | 220 | II | Y | N | N | |||
| 0735 | 38 | 2 | N | 359 | N | N | N | Y | 174 | Y | ||||
| 0743 | 38 | 2 | N | 335 | Y | 45 | Nil | Y | 171 | II | N | N | Y | |
| 0775 | 59 | 2 | N | 311 | N | N | N | Y | 166 | N | ||||
| 0786 | 61 | 2 | N | 288 | N | N | N | N | N | |||||
| 07110 | 68 | 2 | N | 239 | N | N | N | N | N | |||||
| 07141 | 50 | 2 | N | 201 | N | N | N | N | N | |||||
| 07143 | 45 | 2 | N | 214 | Y | 50 101 | Val | Y | 87 | II | Y | N | N | |
| 07138 | 73 | 2 | N | 213 | N | N | N | Y | 137 | N | ||||
| 07169 | 61 | 2 | N | 155 | N | N | N | N | N | |||||
Day indicates day after HSCT; and Val, valciclovir.
Any positive peripheral blood CMV PCR.
Episodes of acute GVHD after T-cell infusion.
Infectious events not associated with any evidence of CMV reactivation.
Includes bone marrow failure day 125 in patient 0702, likely secondary to trimethoprim/sulphamethoxazole, necessitating a second transplantation with death from uncontrolled GVHD on day 239, death from relapse in patient 0735 on day 359, and an episode of hypoxia with uncertain etiology in patient 0743 on day 281.
Incidence of CMV reactivation and requirement for antivirals after T-cell infusion
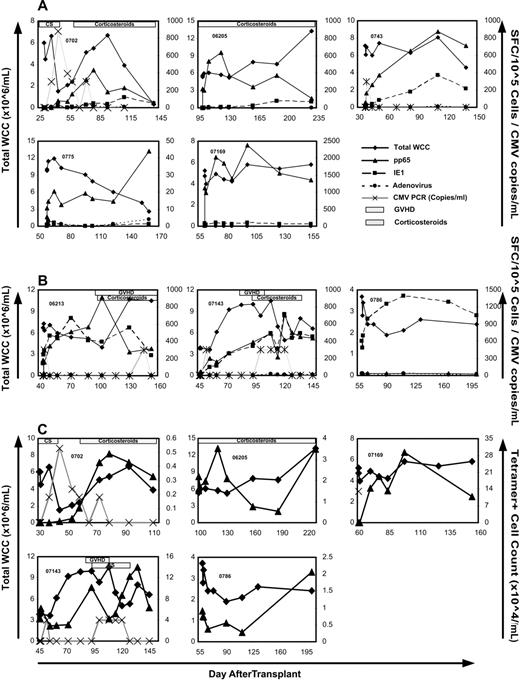
After a median follow-up of 218 days (range, 94-354 days) after T-cell infusion (median, 246 days; range, 135-453 days after transplantation), 6 episodes of CMV reactivation as detected by qualitative and quantitative plasma PCR occurred in 4 patients (Table 2). Four of these episodes consisted of a single-positive PCR with a level of less than 600 copies/mL. Three occurred within the first week after infusion (06213, 0743, and 07143). One reactivation occurred in the context of grade 3 acute GVHD treated with 1 mg/kg per day of prednisolone (06213) 101 days after infusion. In one recipient (0702), there were persistent low levels (maximum 880 copies/mL) of CMV detectable over a 6-week period. This was associated with low-dose (< 0.5 mg/kg per day) oral prednisolone for acute gout. Reactivation was followed by immune reconstitution to CMV, which was predominantly directed at pp65 (compared with IE1; Figure 2A).
CMV-specific immune reconstitution after T-cell infusion. ELISPOT (A,B) and tetramer (C) analysis of response to CMV-pp65 (▴), CMV-IE1 (■), and adenoviral-hexon protein (•) in the trial participants over 3 months after T-cell infusion. Total white cell count (♦) and CMV PCR titer (×) are also shown. Presence of GVHD and corticosteroids is indicated by striped and spotted bars, respectively. Note the individual variation in scale of the y-axis. (A) Five participants with predominantly pp65 immune reconstitution. (B) Three participants with IE1 immune reconstitution matching that of pp65 (2 left panels) or dominating the response (right panel). (C) CMV-pp65 epitope-specific immune reconstitution over 3 months after T-cell infusion as assessed by tetramer binding in 5 patients. Total white cell count (×106/mL) is represented by ♦; tetramer+ cell count (×104/mL) is shown as ▴. CMV PCR titer (×102 copies/mL) is represented by ×. The HLA restriction of the tetramers used included HLA A*0201 (06 205 and 07 169), HLA B*0702 (0702 and 07 143), and HLA B*0801 (0786).
CMV-specific immune reconstitution after T-cell infusion. ELISPOT (A,B) and tetramer (C) analysis of response to CMV-pp65 (▴), CMV-IE1 (■), and adenoviral-hexon protein (•) in the trial participants over 3 months after T-cell infusion. Total white cell count (♦) and CMV PCR titer (×) are also shown. Presence of GVHD and corticosteroids is indicated by striped and spotted bars, respectively. Note the individual variation in scale of the y-axis. (A) Five participants with predominantly pp65 immune reconstitution. (B) Three participants with IE1 immune reconstitution matching that of pp65 (2 left panels) or dominating the response (right panel). (C) CMV-pp65 epitope-specific immune reconstitution over 3 months after T-cell infusion as assessed by tetramer binding in 5 patients. Total white cell count (×106/mL) is represented by ♦; tetramer+ cell count (×104/mL) is shown as ▴. CMV PCR titer (×102 copies/mL) is represented by ×. The HLA restriction of the tetramers used included HLA A*0201 (06 205 and 07 169), HLA B*0702 (0702 and 07 143), and HLA B*0801 (0786).
Patient 07 143 had developed CMV reactivation during induction chemotherapy for AML and had documented reactivation early after HSCT (day 10). Although promptly suppressed by foscarnet, CMV levels rose rapidly with cessation of pharmacotherapy on day 34 with a maximum level of 2810 copies/mL on day 38. Five days after CMV-specific T-cell infusion, a single-positive CMV PCR with copy levels less than 600/mL was recorded, followed by persistently negative results despite absence of foscarnet therapy. A second positive PCR with a level of less than 600 copies/mL was detected 56 days after T-cell infusion associated with corticosteroid therapy (prednisolone 0.5 mg/kg per day) for grade 1 acute GVHD. This low-level DNAemia persisted until prednisolone therapy was reduced to less than 0.1 mg/kg per day.
No patient required foscarnet or ganciclovir therapy. Two patients received high-dose valaciclovir. Patient 0702 was prescribed valaciclovir 1 g 4 times per day 2 weeks after infusion with the intent of suppressing CMV levels. This was continued until 9 weeks after infusion when the dose was reduced to 500 mg per day. Patient 07143 started valaciclovir 2 g 4 times per day 2 weeks after infusion concurrent with immunoglobulin for prevention of acute varicella zoster after exposure to a person with active infection. This continued for 4 weeks, after which the dose was reduced to 500 mg twice per day. Valaciclovir was again briefly increased to 2 g 4 times per day by an independent treating physician when a positive CMV PCR was detected 56 days after T-cell infusion.
CMV-specific immune reconstitution after T-cell infusion
Functional immune reconstitution to whole CMV and adenoviral hexon protein antigens was determined by ELISPOT analysis of samples taken during the first 3 months after T-cell infusion (Figure 2A,B). Epitope-specific reconstitution was also examined using the tetramer analysis (Figure 2C). All 12 demonstrated evidence of CMV-specific immunity of at least one time point when examined by ELISPOT. In 5 of the 12 participants, rapid reconstitution to pp65 occurred with minimal (0702, 06205, and 0743) or no (0775 and 07169) reconstitution of IE1-specific immunity (Figure 2A). In 3 patients, IE1-specific reconstitution either matched pp65 (06213 and 07143) or dominated the immune response (0786) (Figure 2B). In the 4 remaining participants, CMV-specific immunity remained unchanged or decreased after T-cell infusion (data not shown). Of these 4 patients, 2 were CMV-seronegative (07141 and 0735) and 2 were receiving corticosteroids at the time of infusion (07110 and 07138). Patient 07138 already had immunity present before infusion with 735 spot-forming cells per 105 cells. This was similar to peak levels reached in other patients with reconstitution in Figure 2A. There was no reconstitution of adenovirus-specific immunity in any of the 12 patients infused.
Tetramer+ T-cell numbers were seen to rise in 5 of the 12 participants (Figure 2C). Two of these were HLA-A0201+ (06205 and 07169), and 3 were HLA-B0702+ (0702, 07143, and 0786). Four of these patients demonstrated functional reconstitution to pp65 (06205, 07169, 0702, and 07143) and 1 to IE1 (0786) on ELISPOT (Figure 2A,B).
GVHD after T-cell infusion
Four patients developed GVHD a median of 82 days (range, 42-133 days) after T-cell infusion (Table 2). All were associated with subtherapeutic levels of cyclosporin (06213 and 07143) or intentional weaning of immunosuppression (06205 and 0743). Patient 06213 developed grade 3 GVHD affecting the skin and liver 45 days after infusion. This did not resolve with oral prednisolone 1 mg/kg per day but responded to five 50-mg doses of daclizumab over 4 weeks. Another episode of grade 3 GVHD affecting the gastrointestinal tract occurred 149 days after T-cell infusion. This second episode resolved with intravenous methylprednisolone 2 mg/kg per day. In all other patients, GVHD consisted of skin involvement no greater than grade 2 in severity and resolved with oral prednisolone therapy 1 mg/kg per day or less.
Other adverse events after T-cell infusion
One patient (0702) developed graft failure 94 days after infusion (125 days after transplantation) associated with trimethoprim/sulphamethoxazole therapy. This patient had developed neutropenia and thrombocytopenia 1 week after initiating trimethoprim/sulphamethoxazole for Pneumocystis carinii pneumonia prophylaxis early after HSCT. Trimethoprim/sulphamethoxazole was restarted at day 94 after HSCT, mild thrombocytopenia (139 × 109/L) was noted 2 weeks later, and profound pancytopenia was noted at day 125 (white cell count 0.6 × 109/L, hemoglobin 75 g/L, platelet count 9 × 109/L). This necessitated a second transplantation with subsequent uncontrolled GVHD leading to death 177 days after T-cell infusion (208 days after the initial transplantation). We considered the possibility that the cells infused contained an autoreactive clone, which led to this graft failure. We therefore assessed the CMV-specific T cells infused by coculturing with irradiated donor stem cells at a ratio of 1:1 in an interferon-γ ELISPOT assay as described. This showed no interferon production elicited in response to the donor stem cell product.
Three patients had malignant relapse after T-cell infusion (Table 2). Patient 0735, who received a matched sibling transplant for relapsed ALL involving the central nervous system, was diagnosed with relapse at the same site 136 days after infusion (174 days after transplantation) and subsequently died of complications of relapse 321 days after T-cell infusion (day 359 after transplantation). Patient 0775 relapsed with AML 107 days after infusion (166 days after transplantation). Patient 07138 was diagnosed with relapsed AML 64 days after T-cell infusion (day 137 after transplantation). Both patients 0775 and 07138 subsequently received reinduction chemotherapy and donor lymphocyte infusions and are alive in remission 252 and 140 days after T-cell infusion (days 311 and 213 after transplantation), respectively.
Non–CMV-related infection occurred in 3 patients (Table 2). Patient 06205 developed type II herpes simplex on cessation of valaciclovir prophylaxis 112 days after T-cell infusion (213 days after transplantation). Patient 07143 had hemorrhagic cystitis associated with polyomavirus isolated from the urine at the time of T-cell infusion. Cystitis improved over the subsequent week with conservative management. This patient also had 2 episodes of central venous catheter-related septicemia 47 and 97 days after infusion (94 and 144 days after transplantation, respectively), which resolved with antibiotics and line removal. Patient 0743 was admitted to hospital 243 days after T-cell infusion (281 days after transplantation) with hypoxia after symptoms suggestive of a viral respiratory tract infection. No etiology was identified, and the episode resolved with supportive care and prednisolone 1.2 mg/kg per day.
There were 2 deaths as described in the previous 2 paragraphs, 1 from GVHD after second transplantation and another from relapsed ALL.
Discussion
Immunotherapy for the prevention and treatment of CMV after HSCT is a promising alternative to pharmacotherapy. Various in vitro methods to generate CMV-specific T cells have been described, but the number of clinical trials is still relatively small. Different sources of antigen have been used to generate the T-cell product infused.1-4,6,7 Here we have confirmed the safety and efficacy of the approach used by Leen et al,5 generating CMV-pp65–specific T cells for prophylactic infusion in 12 HSCT recipients.
CMV reactivation occurred in 4 of the 12 patients but was of low titer in each case. In these patients, there was a notable lack of need for antivirals, including high-dose valaciclovir, foscarnet, and ganciclovir. Only 2 trial patients received high-dose valaciclovir, and none required foscarnet or ganciclovir. In contrast, during a similar time period to our ongoing immunotherapy trials, 82% of 28 CMV PCR-positive nontrial patients with CMV-seropositive donors received antivirals, and 50% required foscarnet or ganciclovir. A total of 30% (4 of 13) of nontrial patients with CMV reactivation who were initially treated with high-dose valaciclovir went on to receive ganciclovir or foscarnet.
Dominant and rapid pp65-directed immune reconstitution in the presence of minimal or no IE1-directed reconstitution was clearly evident in 5 participants. This is suggestive of the efficacy of the adoptive transfer of CMV-specific immunity in this cohort of patients. In 3 recipients, rapid IE1-directed reconstitution matched that of pp65 or dominated the immune response. This may reflect a normal process after HSCT. However, it is possible that the rapidity of reconstitution may have been the result of CD4+ T-cell help provided by the infused cells. Of the 4 recipients who did not show significant changes to immunity after infusion, 2 were CMV-seronegative and 2 were taking corticosteroids for GVHD.
There were no adverse events related to the product within the 24 hours after infusion. Rates of GVHD, infection, and death were not increased compared with expected rates. This suggests the safety of the currently used cell dose and the possibility of infusing higher cell doses to establish a correlation between cell dose, CMV-specific immune reconstitution, and CMV prevention. Our study did not do this because of ethical concerns about the risk of infusing higher cell doses as prophylaxis in patients without either CMV reactivation or disease.
Significant delays in the planned day of administration were mostly the result of GVHD, corticosteroid use, or cyclosporin toxicity. Because prior GVHD or steroid usage, if anything, increases the risk of postinfusion events, such as GVHD or poor CMV immune reconstitution, the delay in administration is unlikely to account for the good safety profile, low CMV PCR titers, or absence of antiviral use seen in this cohort. These positive benefits could be attributed to low rates of grade III and grade IV GVHD and steroid dosage in the patient group, but this is doubtful to account for all of the benefit seen. Although numerous studies have highlighted the importance of GVHD as a predisposing factor for CMV reactivation,16-18 the role of steroid dosage is considered to be less significant, with at least one early study showing that use of topical, low- or high-dose systemic steroids in GVHD did not impact the rate of reactivation.17 Taken together with the 9 patients in our earlier trial who received T cells stimulated with NLV peptide, a total of 21 patients have received prophylactic CMV-specific T cells at our institution.8 None has required therapeutic foscarnet or ganciclovir, further strengthening these results.
There are several differences between this trial and our previous experience with CMV peptide-specific T-cell infusions in HSCT recipients.8 First, despite all 12 Ad5f35pp65 trial recipients demonstrating some degree of CMV-specific immunity by ELISPOT, single epitope tetramers were only useful for assessing reconstitution in 5 (2 HLA-A0201+ and 3 HLA-B0702+) of the 12 Ad5f35pp65 trial recipients; 6 of the 9 patients receiving CMV peptide-specific T cells showed a rise in the tetramer+ population. This difference is not surprising given the polyepitope-specific nature of cells infused in this current trial and the sensitivity of tetramers used. The second difference is in regards to the persistence of functional immunity in those with CMV-specific reconstitution. In 2 patients receiving CMV peptide-specific T cells, ELISPOT analysis showed a transient rise in functional response to the individual epitope, which mirrored the temporary rise in tetramer+ cell numbers after infusion. In contrast, the 5 recipients in the current trial who had predominantly pp65-specific reconstitution had persistently elevated levels of immunity compared with baseline. This was apparent even in those on corticosteroids. This may reflect the presence of CMV-specific CD4+ T-cell help in these cultures in contrast to the predominantly CD8+ cytototoxic T cells seen in peptide-stimulated cultures.
Although the results of this trial confirm those of the Houston group in regards to prevention of CMV disease, significant differences exist regarding the patient mix, culture technique, and adenovirus-specific immune reconstitution.5 The Houston patient group differed from this one, specifically in terms of younger age (mixed child and adult compared with only adults), higher proportion of transplants from matched unrelated donors (6 of 11 compared with 4 of 12), and use of in vivo T-cell depletion (9 of 11 compared with 4 of 12 in our study). The efficacy seen among these differing populations demonstrates the applicability of this technique to a broad range of patients.
Unlike the cells raised by the Houston group, generation of EBV-specific T cells was not incorporated into this protocol. Our institution has had few problems with posttransplant EBV infections, and the majority of our transplant recipients do not receive T-cell depletion. The advantage of this omission is that it avoids the need for the production of EBV-infected lymphoblastoid cell lines, thus shortening the overall T-cell generation process by several weeks. The simplicity of our culture system makes it easier to implement in smaller yet busy transplant centers with limited resources who may have a case mix similar to ours.
Like the Houston group, small populations of adenovirus-specific T cells were seen in the infusional product of 2 patients examined with adenovirus-specific pentamers. There was also killing of adenovirus pepmix-pulsed target cells above background in 10 of the cultures (with 3 having a specific lysis > 10% and 2 of these > 50%). In contrast to the Houston trial, none of the 12 recipients had prominent reconstitution to adenovirus after infusion. This may be the result of the small number of adenovirus-specific cells present in the infusional product. This could be attributed to the dominance of a CMV response in the culture system in CMV-seropositive donors (C. Bollard, Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX, personal oral communication, November 2005) and might be overcome by addition of a culture well comprising DCs transduced with an empty adenoviral vector. Alternatively, lack of adenovirus-specific reconstitution in vivo could reflect an absence of adenoviral infections in the current trial participants (all adults) compared with the 5 documented infections among the patients in the Houston trial.
The positive results of this trial add to the mounting evidence in favor of incorporating immunotherapy routinely into HSCT. Much work still remains to be done, however. Optimization of techniques is an ongoing process. Recent advances, such as direct isolation of antigen-specific T cells through cytokine secretion or markers of activation, may lead to less manipulation and improved survival of cells infused.19,20 CMV is just one of many organisms that may be amenable to this form of therapy. We are currently examining the incorporation of generation of varicella zoster virus-specific and Aspergillus-specific T cells into routine cultures.
Administration of ex vivo–generated antigen-specific cells remains an experimental approach requiring adherence to principles of good manufacturing practice, clean room facilities, and satisfaction of local regulatory requirements. As laboratories supporting clinical stem cell transplantation conform to regulatory requirements, this form of therapy will become widely available if it can be demonstrated to be clinically beneficial. Its complexity should not be exaggerated. In our trial, cell culture was performed using one stem cell transplantation scientist (L.C.). Alternatively, the outsourcing of tissue culture procedures to a few large centers with concentrated facilities and expertise may be the more cost-effective and rational means of expanding this therapy into the mainstream.
In conclusion, prophylactic infusion of CMV-specific T cells generated using the Ad5f35pp65 vector is safe and appears to be effective in preventing CMV and avoiding the need for anti-CMV pharmacotherapy in HSCT recipients. This trial provides evidence of the efficacy of this technique in a broader range of patients than previously studied.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Malcolm Brenner, Cliona Rooney, Helen Heslop, and Catherine Bollard from the Center for Cell and Gene Therapy, Baylor College of Medicine (Houston, TX) for supplying the Ad5f35pp65 vector and technical advice.
This work was supported in part by the Cancer Council of New South Wales. K.P.M. was supported by the Leukemia Foundation Australia.
Authorship
Contribution: K.P.M. enrolled trial participants, infused the CMV-specific T cells, performed clinical and laboratory postinfusion monitoring, and wrote the paper; L.C. and U.S. generated the CMV-pp65-specific T cells for infusion; A.M.H. optimized culture conditions and wrote the protocols for the generation of CMV-specific T-cell product; E.B. assisted with the clinical follow-up and laboratory monitoring; V.A. assisted with clinical grade cell generation and freezing; M.M.S. assisted with technical advice on flow cytometry; K.F.B. cared for transplant patients and reviewed the manuscript; and D.J.G. designed and established the protocol, oversaw the trial, assisted with postinfusion clinical monitoring and assisted in writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. Gottlieb, Department of Medicine, Westmead Hospital, Sydney, NSW 2145, Australia; e-mail: david_gottlieb@wmi.usyd.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal