Anemia is highly prevalent in children in malaria-endemic areas. However, it is difficult to distinguish between IDA and ACD in affected populations. In this issue of Blood, Atkinson and colleagues identify SNPs in the TNFα gene that are associated with an increased risk of developing IDA during the malaria season.
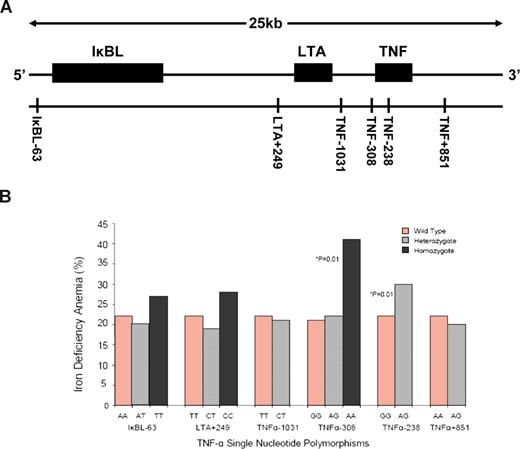
Single nucleotide polymorphisms (SNPs) in the TNF gene locus (lying within the Major Histocompatability Complex class III region on chromosome 6) have been identified as potential risk factors in the etiology of a number of diseases, including malaria. TNF promoter polymorphisms are associated with increased TNF gene transcription, and previous work has provided strong evidence that plasma tumor necrosis factor alpha (TNFα) levels are significantly elevated following malarial infection.1 TNFα is known to be a modifier of body iron status and, in their study, Atkinson et al investigated whether functional SNPs and haplotypes across the TNF gene locus were associated with anemia during the malaria season. A cohort of 780 children was recruited from rural villages in the malaria-endemic West Kiang region of The Gambia. Blood samples were collected from each child at the start (baseline measurement) and end of the malaria season. The samples were used to assess iron status and inflammation as well as to provide genomic DNA for analysis of TNF gene SNPs. The data revealed a significant increase in the prevalence of iron deficiency and iron deficiency anemia (IDA), together with a marginal rise in the incidence of anemia of chronic disease (ACD) over the malaria season. While TNF gene polymorphisms were not significantly associated with aberrant iron status at baseline, individuals with the TNF-308AA genotype had a significantly increased risk of developing iron deficiency and IDA by the end of the malaria season. Similarly, children carrying the TNF-238AG genotype also had a significantly greater risk of IDA, as detailed in the figure. Of the 9 TNF haplotypes identified, only 1 (discriminated by the TNF-308A allele) was associated with increased incidences of iron deficiency and IDA. Interestingly, there was no association between TNF gene SNPs and haplotypes and the development of ACD over the malaria season.
(A). The TNF gene locus showing SNPs spanning a 25kb region across the IκBL, LTA, and TNF loci. (B) Percentage of children with iron deficiency anemia at the end of the malaria season grouped by TNF single nucleotide polymorphisms. See complete figure in the article beginning on page 4276.
(A). The TNF gene locus showing SNPs spanning a 25kb region across the IκBL, LTA, and TNF loci. (B) Percentage of children with iron deficiency anemia at the end of the malaria season grouped by TNF single nucleotide polymorphisms. See complete figure in the article beginning on page 4276.
How might TNF SNPs be related to the development of iron deficiency and IDA? Atkinson et al hypothesize that malarial infection together with TNF polymorphisms significantly increase plasma TNFα levels (they did not measure circulating TNFα concentration in this study). Previous work has shown that TNFα is a powerful inhibitor of iron absorption by the intestinal epithelium,2-4 iron recycling by reticuloendothelial macrophages,5 and erythropoiesis.6,7 These effects are likely to be greatly exacerbated by the fact that the malaria season in The Gambia coincides with the “hungry season” when dietary iron supply is limited.
Interestingly, Atkinson et al also found that children homozygous for a second haplotype distinguished by SNPs in the inhibitory kappa B-like (IκBL) and lymphotoxin alpha (LTA) genes, which lie immediately upstream of TNF, were more likely to be iron replete at the end of the malaria season. Other members of the IκB family of proteins inhibit the actions of the tran-scription factor Nuclear Factor-kappa B (NF-κB), which is required for the transcriptional activation of the TNFα gene. The authors speculate that IκBL might also inhibit NF-κB and thereby diminish the effects of TNFα on intestinal iron absorption and macrophage iron recycling. The possible involvement of the LTA SNP in controlling iron homeostasis is unclear at present.
Despite being portrayed in many studies as a disease risk–associated region, Atkinson et al speculate that there might in fact be potential benefits in carrying SNPs in the TNF gene locus. They suggest that the association between TNF promoter polymorphisms, malaria, and nutritional iron deficiency and IDA may have developed as an evolutionary adaptation to limit iron availability for microorganisms and thereby offer protection against the development of infectious diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal