Abstract
Macrophage mannose receptor (MR) participates in pathogen recognition, clearance of endogenous serum glycoproteins, and antigen presentation. MR is also present on lymphatic vessels, where its function is unknown. Here we show that migration of lymphocytes from the skin into the draining lymph nodes through the afferent lymphatics is reduced in MR-deficient mice, while the structure of lymphatic vasculature remains normal in these animals. Moreover, in a tumor model the primary tumors grow significantly bigger in MR−/− mice than in the wild-type (WT) controls, whereas the regional lymph node metastases are markedly smaller. Adhesion of both normal lymphocytes and tumor cells to lymphatic vessels is significantly decreased in MR-deficient mice. The ability of macrophages to present tumor antigens is indistinguishable between the 2 genotypes. Thus, MR on lymphatic endothelial cells is involved in leukocyte trafficking and contributes to the metastatic behavior of cancer cells. Blocking of MR may provide a new approach to controlling inflammation and cancer metastasis by targeting the lymphatic vasculature.
Introduction
Molecular mechanisms regulating lymphocyte entry from the blood into the lymphoid tissues during their recirculation are well characterized.1,2 In contrast, much less is known about the exit mechanisms of leukocytes from the peripheral tissues into the afferent lymphatics and from the lymphoid organs into efferent lymphatics, although lymphocyte and dendritic cell migration via the lymphatics is essential for controlling the nature and magnitude of immune responses.3 Moreover, lymphatics are important routes for metastasis of different types of cancers. For example, breast cancers and squamocellular cancers in the head and neck area spread mainly via lymphatics.4 In vitro studies have suggested that macrophage mannose receptor (MR) is, in addition to macrophages, abundantly present on both afferent and efferent lymphatics.5-7 Furthermore, MR in the lymphatic tissues of human tissue sections is able to bind lymphocytes and certain types of tumor cells.8,9 However, lack of function-blocking antibodies against mouse MR and relevant gene-manipulated mice have prevented an evaluation of the role of MR in lymphocyte trafficking in vivo. Recent availability of MR−/− mice allowed us to study the role of lymphatic-associated MR
Methods
Animals
Specific pathogen-free age- and sex-matched (6-16 weeks old) wild-type (WT) and MR−/− mice (on the C57BL/6 background) were used in the experiments. Production of MR−/− mice has been described earlier.10 The experiments were approved by the Animal Care Committee of the University of Turku (Turku, Finland) and conformed to the guidelines established by the European Union.
Flow cytometry
The phenotype of lymphocytes from WT and MR−/− mice was determined by flow cytometry. In brief, cell suspensions were prepared from the peripheral (axillary and inguinal) lymph nodes, mesenteric lymph nodes, Peyer patches, thymus, and spleen using mechanical teasing through a steel mesh. Erythrocytes were lysed from the spleen by a brief hypotonic lysis. Cells were stained with phycoerythrin (PE)–conjugated monoclonal antibodies against CD4, CD8, and CD62L (BD Pharmingen, San Diego, CA). Biotinylated B220 antibody (TIB146; ATCC, Manassas, VA) followed by PE-conjugated streptavidin was used to detect B cells. Labeled cells were analyzed by FACScan (BD Biosciences, San Jose, CA).
Lymphocyte migration via lymphatic vessels
Lymphocyte migration into the draining lymph nodes was measured as described.11 In brief, cells from peripheral lymph nodes, mesenteric lymph nodes, and spleen of WT mice were fluorescently labeled by a 30-minute incubation with 5-chloromethyl fluorescein diacetate (CMFDA; Invitrogen, Carlsbad, CA). After washing, 5 × 106 cells were injected subcutaneously into the left hind footpad of MR−/− mice and WT controls. After 13 hours, the recipient mice were killed and lymphocyte suspensions were prepared from popliteal lymph nodes. Cells were then stained with Alexa Fluor 647–conjugated anti-CD4 monoclonal antibody (mAb), peridinin chlorophyll protein–cyanine 5,5 (PerCP-Cy5,5)–conjugated anti-CD8 mAb, and biotinylated B220 antibody followed by PE-conjugated streptavidin. Migration efficiency was determined as percentage of injected cells (CMFDA-labeled) within lymph node lymphocytes by flow cytometry. The 13-hour migration time was pretested to be optimal, as at this time point the injected cells have migrated to and are dispersed throughout the draining lymph node but have not yet left it via the efferent lymphatics.
Tumor cell migration via lymphatic vessels
B16-F10-luc-G5 melanoma cells (Xenogen, Alameda, CA) were injected subcutaneously (4 × 105 in 30 μL RPMI) into the left ear of 19 MR−/− and 19 WT mice. Primary tumor and metastatic growth were monitored twice a week using bioluminescence imaging as described previously.12 In brief, mice were anesthetized with 2.5% isoflurane (Becton Dickinson, Lincoln Park, NJ). A substrate for the luciferase (d-luciferin sodium salt; Synchem, Kassel, Germany; 150 mg/kg intraperitoneally) was injected into mice 10 minutes before imaging. Photographic images were taken in the IVIS imaging station with a cooled CCD camera (IVIS; Xenogen). Signal intensities were quantified from regions of interest as photon counts using the Living Image software (Xenogen).
Immunohistochemistry
Acetone-fixed frozen sections (5 μm) of the ear and peripheral lymph nodes or lymph node metastases of the MR−/− (n = 4) and WT (n = 4) mice were stained with polyclonal rabbit anti–mouse LYVE (Relia Tech, San Pablo, CA) and monoclonal anti-Langerin (CD207; eBioscience, San Diego, CA) or with negative control mAb or serum (anti–human CD44, Hermes-1, and normal rabbit serum). In addition, ears and lymph nodes of 3 MR−/− and 3 WT mice were stained with goat anti–mouse CCL21 (R&D Systems, Minneapolis, MN), rabbit anti–mouse S1P1 (Cayman Chemical, Ann Arbor, MI), rat mAb MECA32 (kind gift from E. Butcher, Stanford University, Stanford, CA), and rat mAb against mouse CD169, clone 3D6, prepared in-house. Fluorescein isothiocyanate (FITC)–conjugated antirabbit, antigoat, or antirat Ig (all from Sigma-Aldrich, St Louis, MO), diluted in phosphate-buffered saline (PBS) containing 5% normal mouse serum, were used as second-stage antibodies. The sections were analyzed using an Olympus BX60 microscope (Hamburg, Germany). Langerin-positive cells were counted in epidermis and dermis in the skin of the ears, in T-cell areas of lymph nodes (because they are practically absent from the B-cell follicles13 ), and in primary tumors and metastases. MR expression was analyzed using MR5D3 antibody14 followed by Alexa Fluor 546–conjugated antirat Ig in immunofluorescence stainings and peroxidase-conjugated rabbit antirat Ig (Dako, Glostrup, Denmark) in immunoperoxidase stainings. For peroxidase-conjugated secondary reagents, 3,3′-diaminobenzidine in PBS containing 0.03% hydrogen peroxide was used as a chromogen.
Microlymphangiography
The collecting lymphatic vessels were visualized by fluorescent microlymphangiography.15 In brief, 5 μL FITC-dextran (MW 2 000 000; 8 mg/mL in PBS; Sigma-Aldrich) was injected into the subepidermal layer in the tip of both ears of 6 normal MR−/− and 6 WT mice. In addition, 6 MR−/− and 6 WT controls were inoculated with B16 melanoma cells into the ear. After 2 weeks, the animals were used for microlymphangiography as explained above in this paragraph. The ears were examined microscopically with an Olympus BX60 equipped with fluorescence light source (Olympus U-RFL-T). The vessel diameters were analyzed using ImageJ image analysis program (public domain; National Institutes of Health [NIH], Bethesda, MD). The vessel densities were calculated as the percentage of the FITC-positive area per field with the same program.
Lymphocyte proliferation assay
The antigen was prepared by lysing B16-luc cells (2 × 106 cell/mL in PBS) by 5 freeze-thaw cycles followed by sonication. B16-luc cell lysate (0.5 mL) was injected intraperitoneally into 8 MR−/− and 8 WT mice. Four MR−/− and 4 WT mice were left as controls. After 7 days, immunized and nonimmunized mice were killed and peritoneal macrophages were harvested. Spleens of the immunized mice were collected for isolation of T cells. Spleens were homogenized and red cells were lysed using hypotonic saline. T cells (0.2 × 106) from immunized MR−/− and WT mice were cocultured at a 10:1 ratio with MR−/− and WT macrophages in round-bottom 96-well plates. Cultures containing T cells and macrophages from nonimmunized MR−/− and WT mice served as baseline controls for proliferation. Cocultures were incubated in complete medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 20 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, penicillin/streptomycin) for 3 days and pulsed with 3H-thymidine (1 μCi [0.037 MBq] per well) for the final 6 hours. Cells were harvested using semiautomated plate harvester (Tomtech MACH III; Fisher Scientific, Hampton, NH) and counted with the 1450 Microbeta counter (Wallac, Turku, Finland).
In vitro adhesion and migration assays
Adhesion assays were performed on frozen sections of lymph nodes of MR−/− and WT mice as described.8 Briefly, mixtures of lymphocytes isolated from mesenteric lymph nodes of WT mice and B16 melanoma cells were incubated for 15 minutes in static conditions, followed by 5 minutes of rotation at 60 rpm and then again 15 minutes without rotation at 7°C. The adherent cells were fixed in 1% glutaraldehyde. Binding of melanoma cells and lymphocytes (easily discriminated by their size) to lymphatic sinuses and high endothelial venules (HEVs) was counted and the results are expressed as number of cells bound/lymphatic sinuses or HEVs (mean ± SEM).
Detection of MR ligands in tumor cell lines
Binding of MR-Fc chimeras to different murine tumor cell lines (F9 teratocarcinoma, EL-4 T lymphoma, 38C13 B lymphoma, LLC-1 Lewis lung cell carcinoma, B16 melanoma, SP2/0 myeloma, MC57G fibrosarcoma, and TC-1 lung epithelial cell tumor) was also tested. The Fc chimeras CR-Fc, CRD4-7-Fc, and CR-FNII-CTLD1-3-Fc16,17 (10 μg/mL in RPMI) were incubated with 106 tumor cells. After 3 washes, the bound chimeras were detected with FITC-anti–human Ig containing 5% normal mouse serum. The cells were analyzed with FacsCalibur (Becton Dickinson).
Cell migration assays
For transwell migration assays, cells from peripheral and mesenteric lymph nodes of MR−/− and WT mice were fluorescently labeled with carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Invitrogen). Different number of cells (from 4 to 25 × 104) were resuspended in RPMI medium and placed into the upper chamber of transwell (96 wells, 3-μm pores; NeuroProbe, Gaithersburg, MD). RPMI with or without murine CCL21 (100 ng/mL; R&D Systems), or with 50% of serum of 4 MR−/− or 4 WT mice was added into the lower chamber. After 2 hours at 37°C, the upper chamber was removed and the number of migrated cells in the lower chamber was determined using Tecan Infinite M200 (Maennedorf, Switzerland).
Statistics
Data were analyzed by the parametric Student t tests. Growth curves were analyzed by repeated measures for analyses of variance. Statistical significance was set at P less than .05.
Results
MR on lymphatics regulates the traffic of both B and T cells into the draining lymph nodes
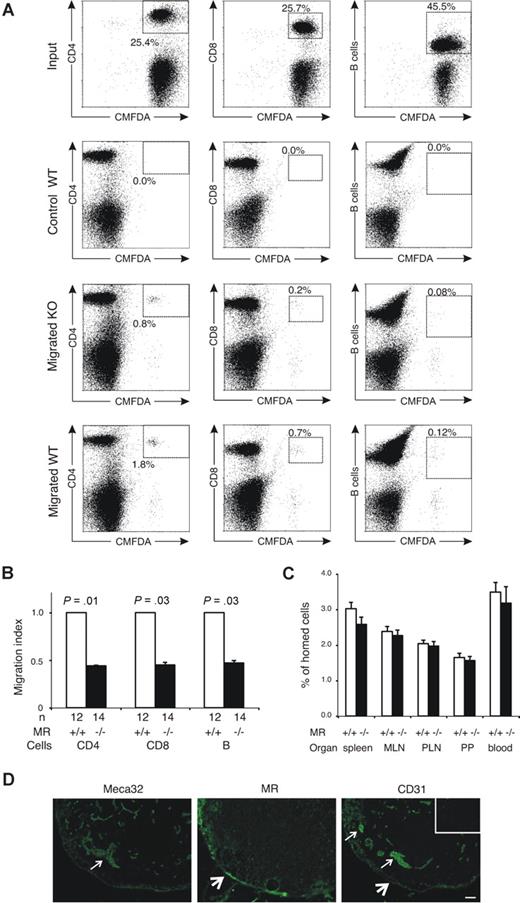
To evaluate the role of MR in lymphocyte migration via afferent lymphatics, we quantified the migration of CMFDA-labeled WT lymphocytes to popliteal lymph nodes after subcutaneous injection into the footpads of both MR−/− and WT mice. We found that the absence of MR significantly impaired the trafficking of CD4+ and CD8+ cells and B cells into the draining lymph nodes (Figure 1A,B). In contrast, short-term in vivo homing experiments analyzing extravasation of intravenously administered lymphocytes from the blood into several lymphatic organs (peripheral lymph nodes, mesenteric lymph nodes, Peyer patches, and spleen) revealed no significant differences between the genotypes (Figure 1C). These results are compatible with the fact that blood vessels are devoid of MR (Figure 1D) and show that lymphocyte extravasation is not markedly affected in these mice. Moreover, the proportions of CD4+ and CD8+ T cells and B220-positive B cells in the lymphoid organs were virtually identical in MR-deficient and WT animals, and even though L-selectin has been shown to be one of the ligands of MR there were no differences in the number of cells expressing this lymphocyte homing receptor (Table 1). These data indicate that MR is involved in the trafficking of T and B cells through the afferent lymphatics but not in lymphocyte homing via blood vessels.
Lack of mannose receptor affects lymphocyte traffic into the draining lymph nodes but not lymphocyte homing to lymphoid organs from the blood. (A,B) CMFDA-labeled lymphocytes were injected into the footpads of MR−/− mice and their controls. The input population and lymphocytes from draining the popliteal lymph node were stained with antibodies against CD4, CD8, and B cells, and the percentage of CMFDA-positive CD4, CD8, and B cells was analyzed with a flow cytometer. (A) Representative fluorescence-activated cell sorting (FACS) blots of injected cells (input) and migrated cells recovered from popliteal lymph nodes of one KO and one WT mouse. The blots obtained from a noninjected contralateral lymph node of a WT mouse are shown as a control (control WT). The percentages of CMFDA-positive cells expressing the indicated marker are shown in the boxes. (B) Combined results (mean ± SEM) of all mice analyzed are shown as relative migration index (number of migrated cells in WT mice is 1.0 by definition). (C) Homing of intravenously injected, CMFDA-labeled lymphocytes after 4-hour recirculation time into the indicated organs. The values shown are mean percentages (± SEM) of the homed cells recovered from individual organs. (D) Staining of serial sections with MECA-32 mAb (a pan–vascular endothelial marker against PV-1 antigen), anti-MR mAb, and anti-CD31. Arrows point to some of the blood vessels, which are positive for MECA-32 and CD31 and negative for MR. Thick arrows point to lymphatic sinuses positive for MR and CD31. Staining with a negative control antibody is shown in the insert. Bar represents 50 μm. Objective: Olympus UPlanFl 10×/0.30 Ph1.
Lack of mannose receptor affects lymphocyte traffic into the draining lymph nodes but not lymphocyte homing to lymphoid organs from the blood. (A,B) CMFDA-labeled lymphocytes were injected into the footpads of MR−/− mice and their controls. The input population and lymphocytes from draining the popliteal lymph node were stained with antibodies against CD4, CD8, and B cells, and the percentage of CMFDA-positive CD4, CD8, and B cells was analyzed with a flow cytometer. (A) Representative fluorescence-activated cell sorting (FACS) blots of injected cells (input) and migrated cells recovered from popliteal lymph nodes of one KO and one WT mouse. The blots obtained from a noninjected contralateral lymph node of a WT mouse are shown as a control (control WT). The percentages of CMFDA-positive cells expressing the indicated marker are shown in the boxes. (B) Combined results (mean ± SEM) of all mice analyzed are shown as relative migration index (number of migrated cells in WT mice is 1.0 by definition). (C) Homing of intravenously injected, CMFDA-labeled lymphocytes after 4-hour recirculation time into the indicated organs. The values shown are mean percentages (± SEM) of the homed cells recovered from individual organs. (D) Staining of serial sections with MECA-32 mAb (a pan–vascular endothelial marker against PV-1 antigen), anti-MR mAb, and anti-CD31. Arrows point to some of the blood vessels, which are positive for MECA-32 and CD31 and negative for MR. Thick arrows point to lymphatic sinuses positive for MR and CD31. Staining with a negative control antibody is shown in the insert. Bar represents 50 μm. Objective: Olympus UPlanFl 10×/0.30 Ph1.
Lymphocyte phenotypes of the mice
| . | MR+/+, % . | MR−/−, % . |
|---|---|---|
| Thymus | ||
| CD4 | 97 ± 0.2 | 97 ± 0.3 |
| CD8 | 92 ± 0.3 | 92 ± 0.7 |
| B220 | 0.4 ± 0.2 | 1 ± 0.2 |
| CD62L | 91 ± 2 | 92 ± 1 |
| Peripheral lymph node | ||
| CD4 | 30 ± 2 | 31 ± 1 |
| CD8 | 30 ± 2 | 28 ± 0.4 |
| B220 | 29 ± 2 | 39 ± 7 |
| CD62L | 83 ± 2 | 86 ± 1 |
| Mesenteric lymph node | ||
| CD4 | 29 ± 2 | 29 ± 1 |
| CD8 | 24 ± 2 | 23 ± 1 |
| B220 | 37 ± 3 | 39 ± 2 |
| CD62L | 83 ± 1 | 85 ± 1 |
| Peyer patch | ||
| CD4 | 10 ± 0.5 | 10 ± 1 |
| CD8 | 2 ± 0.3 | 2 ± 0.2 |
| B220 | 83 ± 1 | 81 ± 1 |
| CD62L | 44 ± 3 | 45 ± 4 |
| Spleen | ||
| CD4 | 12 ± 2 | 14 ± 2 |
| CD8 | 4 ± 1 | 5 ± 1 |
| B220 | 74 ± 3 | 70 ± 2 |
| CD62L | 71 ± 5 | 79 ± 3 |
| . | MR+/+, % . | MR−/−, % . |
|---|---|---|
| Thymus | ||
| CD4 | 97 ± 0.2 | 97 ± 0.3 |
| CD8 | 92 ± 0.3 | 92 ± 0.7 |
| B220 | 0.4 ± 0.2 | 1 ± 0.2 |
| CD62L | 91 ± 2 | 92 ± 1 |
| Peripheral lymph node | ||
| CD4 | 30 ± 2 | 31 ± 1 |
| CD8 | 30 ± 2 | 28 ± 0.4 |
| B220 | 29 ± 2 | 39 ± 7 |
| CD62L | 83 ± 2 | 86 ± 1 |
| Mesenteric lymph node | ||
| CD4 | 29 ± 2 | 29 ± 1 |
| CD8 | 24 ± 2 | 23 ± 1 |
| B220 | 37 ± 3 | 39 ± 2 |
| CD62L | 83 ± 1 | 85 ± 1 |
| Peyer patch | ||
| CD4 | 10 ± 0.5 | 10 ± 1 |
| CD8 | 2 ± 0.3 | 2 ± 0.2 |
| B220 | 83 ± 1 | 81 ± 1 |
| CD62L | 44 ± 3 | 45 ± 4 |
| Spleen | ||
| CD4 | 12 ± 2 | 14 ± 2 |
| CD8 | 4 ± 1 | 5 ± 1 |
| B220 | 74 ± 3 | 70 ± 2 |
| CD62L | 71 ± 5 | 79 ± 3 |
Values are means plus or minus SEM. None of the values is statistically different between the groups except B220 in thymus (P < .05). n is 8 in each group.
MR is responsible for tumor metastasis via lymphatics
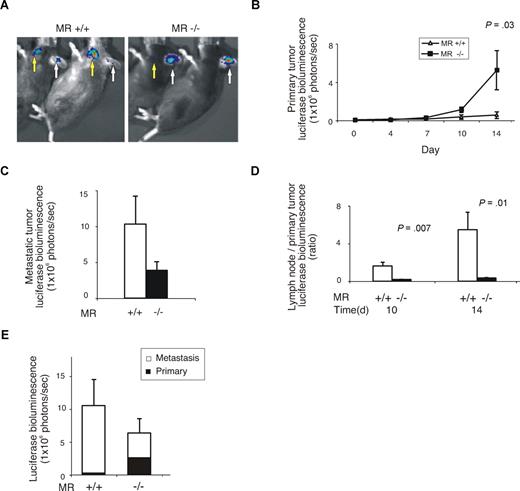
Many tumors spread via the lymphatic vessels.18,19 To study the role of MR in the metastatic behavior of cancer cells we used an in vivo model that measures local growth and lymphatic spreading of tumor cells. This model involves the injection of melanoma cells subcutaneously into the ear, which results in metastatic spread to the draining lymph nodes in the neck. By using luciferase-expressing melanoma cells, the growth of primary tumor and metastatic lesions could be assessed in real time in living animals using bioluminescence imaging (Figure 2A). In WT animals, the luciferase signal (that correlates to the number of living tumor cells) from the primary tumor increased on average 8.5-fold during the 2-week follow-up. Strikingly, we found an average increase of 89-fold in the signal in MR-deficient animals, indicating much faster growth of the primary tumors (Figure 2B). In contrast, the metastatic lesions in the draining lymph nodes were much smaller in the MR−/− animals than in the WT controls at the 2-week time point (Figure 2C). Similarly, the ratio between the sizes of the lymph node metastases and primary tumors was significantly smaller in MR−/− than in the control mice (Figure 2D). Importantly, the overall growth of the tumor foci (primary + metastasis) was not higher in MR−/− than in control mice even though the location of the growth was markedly different (Figure 2E). Collectively, these data suggest that the lack of MR leads not only to faster growth of the primary tumor but also impairs the formation of metastatic lesions in the draining lymph nodes.
Lack of mannose receptor alters the behavior of B16 melanoma. (A) Representative examples of mice after subcutaneous injections of B16 melanoma cells bearing the luciferase-containing construct into the ears. Arrows point to the signals from the primary tumors (white) and metastatic foci (yellow) in the neck lymph nodes. (B) The size of the primary tumors during the 14-day follow-up measured based on the luciferase signals. (C) The size of the lymph node metastases at the end of the experiment. (D) The ratios between the lymph node metastases and primary tumors of WT and MR−/− mice. (E) Total luciferase counts of primary tumor and lymph node metastases in MR−/− and WT mice at the end of the experiments (14 days). All values shown are means (± SEM).
Lack of mannose receptor alters the behavior of B16 melanoma. (A) Representative examples of mice after subcutaneous injections of B16 melanoma cells bearing the luciferase-containing construct into the ears. Arrows point to the signals from the primary tumors (white) and metastatic foci (yellow) in the neck lymph nodes. (B) The size of the primary tumors during the 14-day follow-up measured based on the luciferase signals. (C) The size of the lymph node metastases at the end of the experiment. (D) The ratios between the lymph node metastases and primary tumors of WT and MR−/− mice. (E) Total luciferase counts of primary tumor and lymph node metastases in MR−/− and WT mice at the end of the experiments (14 days). All values shown are means (± SEM).
To study whether lymphocytes of MR−/− and control mice respond differently to CCL21 chemokine or whether sera from these animals have different capacity to attract lymphocytes, we performed transwell migration assays. In these assays we did not observe any differences between the lymphocytes or sera of MR−/− and WT mice (data not shown). These data support the idea that adhesion defects rather than chemotactic alterations are behind the impaired migration of lymphocytes and melanoma cells into the draining lymph nodes.
Tumors of MR−/− and WT mice contain comparable number of dendritic cells
One possible reason for the aberrant growth pattern of the tumors in MR−/− mice could be impaired migration of dendritic cells from the skin to the draining lymph nodes, thereby reducing presentation of tumor antigens to lymphocytes. Therefore, we tested whether the number of dendritic cells originating from skin is altered in MR deficient mice. Nonchallenged MR−/− mice and their controls had comparable numbers of dendritic cells in the epidermis and dermis of the ears when detected with anti-Langerin antibody (Figure 3A). Most Langerin-positive cells were located in epidermis, but occasionally they were also found in dermis. However, approximately 30% fewer Langerin-positive cells were seen in the draining lymph nodes of MRKO mice compared with those of WT animals, although the difference did not reach statistical significance (Figure 3B). Tumors (primary and metastases) of both genotypes had very few Langerin-positive cells, and these were randomly dispersed throughout the tumors in comparable numbers (Figure 3B, data from metastases are shown). Thus, the numbers of dendritic cells present in primary and metastatic tumor foci cannot explain the aberrant growth pattern of B16 melanoma in MR−/− mice.
Number of dendritic cells and presentation of tumor antigens by macrophages are normal in MR−/− mice. (A) Langerin-positive cells were counted in the ears and lymph nodes in non–tumor-bearing and tumor-bearing MR−/− and WT mice (n = 4 in all groups). In tumor-bearing mice, the Langerin-positive cells were counted in non–tumor-containing area. The values are means (± SEM) per mm of epithelial surface of the ears. (B) Langerin-positive cells in lymph nodes (LNs) of non–tumor-bearing mice and in lymph node metastases of mice with tumors. The values are means (± SEM) per mm2 for the lymph nodes/metastases. (C) The ability of macrophages (MOs) to present tumor antigen was measured using proliferation assays. Different combinations of macrophages and T cells from MR−/− and WT mice were used as indicated in the figure. The results are expressed as means (± SEM).
Number of dendritic cells and presentation of tumor antigens by macrophages are normal in MR−/− mice. (A) Langerin-positive cells were counted in the ears and lymph nodes in non–tumor-bearing and tumor-bearing MR−/− and WT mice (n = 4 in all groups). In tumor-bearing mice, the Langerin-positive cells were counted in non–tumor-containing area. The values are means (± SEM) per mm of epithelial surface of the ears. (B) Langerin-positive cells in lymph nodes (LNs) of non–tumor-bearing mice and in lymph node metastases of mice with tumors. The values are means (± SEM) per mm2 for the lymph nodes/metastases. (C) The ability of macrophages (MOs) to present tumor antigen was measured using proliferation assays. Different combinations of macrophages and T cells from MR−/− and WT mice were used as indicated in the figure. The results are expressed as means (± SEM).
Macrophages of MR−/− mice are able to normal presentation of tumor antigen
As macrophage mannose receptor is present on macrophages and dendritic cells and could be potentially involved in antitumor immune response, we analyzed the capacity of peritoneal macrophages to present tumor antigens. After in vivo immunization of MR−/− and WT mice, in vitro proliferation assays were performed using different combinations of macrophages and T cells isolated from these mice. These assays showed that lack of MR does not affect the ability of in vivo–primed macrophages to present tumor antigens to T cells (Figure 3C).
Normal density and morphology of lymphatics in MR-deficient mice
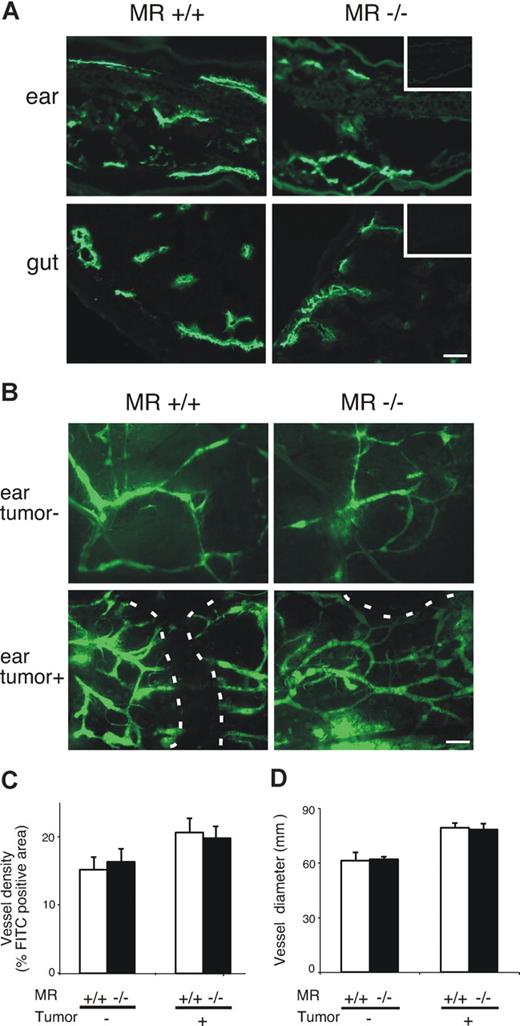
Because MR is present on the lymphatic endothelium, its deletion could affect the structure or fluid-collecting function of the lymphatic vasculature. Immunostaining with LYVE-1, a lymphatic endothelium selective marker, showed that the number and overall morphology of lymphatic vessels were similar in WT and MR−/− animals (Figure 4A). The expression patterns of sphingosine 1 phosphate receptor 1 (S1P1) and CCL21, 2 molecules involved in lymphatic migration, were also indistinguishable between WT and MR−/− mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, expression of CD169/sialoadhesin, identifying macrophages in subcapsular sinus, was similar in both genotypes (Figure S1).
Lymphatics are anatomically and phenotypically normal in MR−/− mice. (A) Examples of expression of LYVE-1 in the ears and gut of normal MR−/− mice and their WT controls. Insets are negative control stainings with normal rabbit serum as the first-stage antibody. Bar represents 50 μm. (B) FITC-dextran was used to visualize lymphatics in the ears of healthy and tumor-bearing MR−/− and WT mice. Lymphatics are almost exclusively peritumoral in the tumor-bearing ears. A dashed line outlines the tumor. Bar represents 100 μm. Objectives: Olympus UPlanFl 10×/0.30 Ph 1 for panel A and 4×/0.13 for panel B. (C) Density and (D) diameter of lymphatics in MR and WT mice without and with tumors. Values shown are means (± SEM).
Lymphatics are anatomically and phenotypically normal in MR−/− mice. (A) Examples of expression of LYVE-1 in the ears and gut of normal MR−/− mice and their WT controls. Insets are negative control stainings with normal rabbit serum as the first-stage antibody. Bar represents 50 μm. (B) FITC-dextran was used to visualize lymphatics in the ears of healthy and tumor-bearing MR−/− and WT mice. Lymphatics are almost exclusively peritumoral in the tumor-bearing ears. A dashed line outlines the tumor. Bar represents 100 μm. Objectives: Olympus UPlanFl 10×/0.30 Ph 1 for panel A and 4×/0.13 for panel B. (C) Density and (D) diameter of lymphatics in MR and WT mice without and with tumors. Values shown are means (± SEM).
Microlymphangiography with subcutaneously injected, high-molecular-weight fluorescent dextran also revealed no detectable differences regarding morphology, diameter, location, and density of lymphatic vessels between WT and MR−/− animals (Figure 4B-D). In tumor-bearing ears, the density of the lymphatics was slightly increased. Practically all lymphatics were peritumoral as no FITC-dextran was visualized within the tumors, but again no differences were evident between the 2 genotypes (Figure 4B,C). Because the absence of MR did not have marked consequences to the morphology or phenotype of lymphatics, these data support the idea that the deficient trafficking of cells in afferent lymphatics in MR−/− animals is caused by lack of the binding between MR and its counterreceptor on migrating cells.
Adhesion of tumor cells and lymphocytes to lymphatic vessels is compromised in MR−/− mice
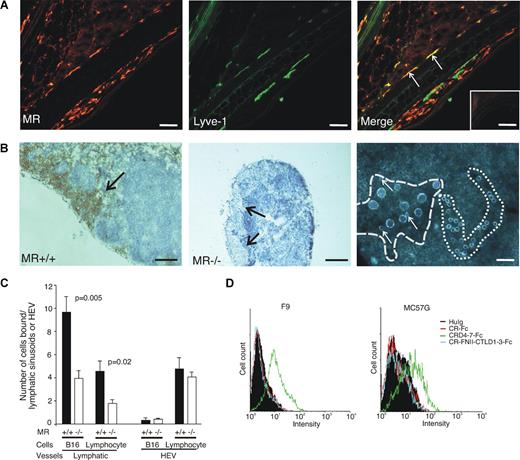
To directly test whether lack of MR affects the binding of lymphocytes and tumor cells to lymphatics, we performed in vitro adhesion assays. Although both afferent and efferent lymphatics are MR positive (Figure 5A,B), afferent lymphatics are very narrow and it is practically impossible to measure adhesion to them. Therefore, we analyzed lymphocyte and tumor cell binding to efferent lymphatic sinuses in lymph nodes. Both tumor cells and lymphocytes avidly adhered to efferent lymphatic sinuses (Figure 5B,C). In contrast, tumor cells showed poor binding to HEVs, while small lymphocytes bound relatively well to HEVs in these assay conditions, which have been optimized to support binding to lymphatic sinuses. Lack of MR significantly reduced binding of both tumor cells and lymphocytes to lymphatic sinuses, but did not affect binding to HEVs (Figure 5C). These data suggest that in the absence of MR the cells cannot efficiently bind to lymphatic sinuses and thus would have impaired capacity to leave the tissues.
Binding of lymphocytes and cancer cells to lymphatic sinuses is compromised in MR−/− mice. (A) Double staining of the WT ear with an anti-MR antibody (MR5D3, red color, left) and anti–LYVE-1 antibody (green color, middle) shows that some of the afferent lymphatics are positive for both markers (arrows pointing out yellow lymphatics in the merge, right). Many MR+LYVE− macrophages are also visible. Negative control staining with class-matched negative control antibodies is shown in the inset. (B) Lymphatic sinuses (dark brown) are MR positive in WT mice (arrow, left), while MR−/− mice completely lack the molecule (arrows, middle). Lymphocytes (small round cells) bind well to HEVs, whereas tumor cells (big cells) show efficient binding only to lymphatic sinuses. The basement membrane outlining an HEV is marked with … and a lymphatic sinus is marked with  in this dark field microscopic micrograph. Three small lymphocytes binding with 9 tumor cells to the lymphatic sinus are pointed out by arrows. Because the adherent cells are lying on the top of the tissue section, the focus of the photograph is a compromise between the tissue and adherent cells. (C) Quantification of the binding of tumor cells and lymphocytes to lymphatic sinuses and HEVs in lymph nodes of MR−/− and WT mice. The results are expressed as number of cells bound to HEVs and lymphatic sinusoids (means ± SEM, n = 6). Bars for panels A and B (right) represent 50 μm and for panel B (left and middle) 0.25 mm. Objectives: Olympus UPlanFl 20×/0.50 Ph 1 for panels A and B (right) and 4×/0.13 for panel B (left and middle). (D) FACS histograms of F9 and MC57G tumor cell lines stained with a negative control (huIg) and different MR-Fc chimeras.
in this dark field microscopic micrograph. Three small lymphocytes binding with 9 tumor cells to the lymphatic sinus are pointed out by arrows. Because the adherent cells are lying on the top of the tissue section, the focus of the photograph is a compromise between the tissue and adherent cells. (C) Quantification of the binding of tumor cells and lymphocytes to lymphatic sinuses and HEVs in lymph nodes of MR−/− and WT mice. The results are expressed as number of cells bound to HEVs and lymphatic sinusoids (means ± SEM, n = 6). Bars for panels A and B (right) represent 50 μm and for panel B (left and middle) 0.25 mm. Objectives: Olympus UPlanFl 20×/0.50 Ph 1 for panels A and B (right) and 4×/0.13 for panel B (left and middle). (D) FACS histograms of F9 and MC57G tumor cell lines stained with a negative control (huIg) and different MR-Fc chimeras.
Binding of lymphocytes and cancer cells to lymphatic sinuses is compromised in MR−/− mice. (A) Double staining of the WT ear with an anti-MR antibody (MR5D3, red color, left) and anti–LYVE-1 antibody (green color, middle) shows that some of the afferent lymphatics are positive for both markers (arrows pointing out yellow lymphatics in the merge, right). Many MR+LYVE− macrophages are also visible. Negative control staining with class-matched negative control antibodies is shown in the inset. (B) Lymphatic sinuses (dark brown) are MR positive in WT mice (arrow, left), while MR−/− mice completely lack the molecule (arrows, middle). Lymphocytes (small round cells) bind well to HEVs, whereas tumor cells (big cells) show efficient binding only to lymphatic sinuses. The basement membrane outlining an HEV is marked with … and a lymphatic sinus is marked with  in this dark field microscopic micrograph. Three small lymphocytes binding with 9 tumor cells to the lymphatic sinus are pointed out by arrows. Because the adherent cells are lying on the top of the tissue section, the focus of the photograph is a compromise between the tissue and adherent cells. (C) Quantification of the binding of tumor cells and lymphocytes to lymphatic sinuses and HEVs in lymph nodes of MR−/− and WT mice. The results are expressed as number of cells bound to HEVs and lymphatic sinusoids (means ± SEM, n = 6). Bars for panels A and B (right) represent 50 μm and for panel B (left and middle) 0.25 mm. Objectives: Olympus UPlanFl 20×/0.50 Ph 1 for panels A and B (right) and 4×/0.13 for panel B (left and middle). (D) FACS histograms of F9 and MC57G tumor cell lines stained with a negative control (huIg) and different MR-Fc chimeras.
in this dark field microscopic micrograph. Three small lymphocytes binding with 9 tumor cells to the lymphatic sinus are pointed out by arrows. Because the adherent cells are lying on the top of the tissue section, the focus of the photograph is a compromise between the tissue and adherent cells. (C) Quantification of the binding of tumor cells and lymphocytes to lymphatic sinuses and HEVs in lymph nodes of MR−/− and WT mice. The results are expressed as number of cells bound to HEVs and lymphatic sinusoids (means ± SEM, n = 6). Bars for panels A and B (right) represent 50 μm and for panel B (left and middle) 0.25 mm. Objectives: Olympus UPlanFl 20×/0.50 Ph 1 for panels A and B (right) and 4×/0.13 for panel B (left and middle). (D) FACS histograms of F9 and MC57G tumor cell lines stained with a negative control (huIg) and different MR-Fc chimeras.
To analyze how commonly tumor cells possess the ability to bind MR, we tested binding of MR-Fc chimeras to 8 different types of tumor cell lines. Five lines (MC57G, EL-4, SP2/0, F9, and B16) showed reproducible staining with the chimera-containing CRD4-7 region (stainings of F9 and MC57G are shown as examples in Figure 5D), whereas very weak or no binding at all was seen to the other 3 lines tested by any of the chimeras. Thus, the possibility to interact with MR is relatively common among tumor types and is not restricted to melanoma.
Discussion
This study demonstrates the importance of MR in the trafficking of different lymphocyte subsets from the periphery via the afferent lymphatics into the draining lymph nodes. Moreover, our results clearly suggest that tumor cells cannot efficiently metastasize to the local lymph nodes in the absence of MR on afferent lymphatics and, consequently, tumors reach bigger sizes at the primary site of injection. Despite being involved in Ag presentation and clearance, MR does not seem to play a role in limiting the growth of the tumor, as the total tumor burden (primary + metastases) does not statistically differ between MR-deficient and WT animals.
MR is present on both afferent and efferent lymphatics.5 Lymphatic sinuses belong to the efferent arm of the lymphatic system and are the exit sites for lymphocytes from the nodes. They are also considered as sieves for arresting cellular debris and microbes.7 This is well in line with the fact that, especially in the mouse, the lymph node sinuses contain an abundant MR-positive macrophage population.6 Theoretically, it would be possible that these medullary MR-positive macrophages are also able to arrest lymphocytes and cancer cells. Our studies favor the idea that MR can serve as an adhesion molecule during cell migration to lymph nodes. We have shown that both lymphocytes and cancer cells bind to lymphatic sinuses in an MR-dependent fashion in vitro. Moreover, in vivo we find no evidence for altered chemotaxis, cell motility, or lymph node entrapment in MR−/− mice. Theoretically, however, it remains possible that some non–adhesion-dependent function could explain the observed phenotype. In any case, we believe that cell migration to lymphatics may be a multistep cascade reminiscent of that seen in the blood vessels and MR is only one step in the continuum of adhesive and chemoattractive events.
Cells may use multiple receptors for binding to MR on lymphatic endothelial cells. L-selectin, which can bind to MR expressed on efferent lymphatic sinuses in vitro,8,20 may also be used by L-selectin–positive lymphocytes when they leave the peripheral tissues via afferent lymphatics. However, our phenotypic analysis of lymphoid tissues showed no marked differences in L-selectin expression between the WT and MR−/− animals (Table 1). These data suggest that additional receptors must exist or that lymphocytes using MR-dependent and MR-independent lymphatic trafficking pathways express similar levels of L-selectin. Moreover, L-selectin–negative lymphocytes and tumor cells must express an additional receptor for MR. Because MR has 3 main types of binding domains (a cysteine-rich domain, a fibronectin type II domain, and 8 C-type lectin-like domains), there are many possibilities for the counterreceptor. Sulfated glycoproteins binding to the cysteine-rich domain and molecules with terminal mannose, fucose, N-acetylglucosamine, or glucose residues binding to carbohydrate recognition domains remain the strongest candidates.8,21-24
MR belongs to pattern recognition molecules and binds various bacteria and yeast cells containing terminal mannose or N-acetylglucosamine on their cell surface. Numerous studies have reported its scavenger function during pathogen elimination, and it is thought to serve a homeostatic function by clearing endogenous glycoproteins.25-27 On the other hand, MR on monocyte-derived dendritic cells has been demonstrated to mediate uptake and presentation of mannosylated antigens.28,29 Interestingly, the MR−/− mice do show impaired clearance of proteins having accessible mannose and N-acetylglucosamine residues, but they mount normal immune response to the pathogens tested.10 These data, when taken together with our study showing normal antigen presentation of tumor antigens in MR−/− mice, may indicate that MR is not required for antigen presentation in vivo or that its function can be compensated by other professional molecules capable of antigen presentation.
Lymphangiogenesis during development and in pathologic conditions in adults has been dissected in great detail for the past few years, and many of the molecules involved have been thoroughly characterized.19 In contrast, the molecular complexity behind the regulation of cell movement, especially that of lymphocytes, into the lymphatics remains largely unresolved, although the importance of lymphatics as an active element in normal leukocyte trafficking and tumor spread is widely accepted.
Recently, several molecular interactions have been reported to contribute to dendritic cell trafficking through afferent lymphatics.30-34 Regarding lymphocyte migration within lymphatics, it is known that, besides attracting dendritic cells, chemokine CCL21 present on lymphatic endothelium guides CCR7-positive lymphocytes into the afferent lymphatics and hence to the draining lymph nodes.11,35 S1P1 expressed in many different cell types participates in lymphocyte exit from the lymph nodes and regulates emigration of thymocytes from the thymus.36-39 Current studies suggest that SIP1 controls lymphocyte trafficking by regulating endothelial permeability and/or suppressing lymphocyte chemotaxis in SIP gradients.39 Compared with MR, the molecules so far described to mediate either lymphocyte or dendritic cell trafficking within the lymphatics have notable differences. They are chemotactic molecules such as SIP1, CCL21, and CXCL12 and/or are expressed on a wide variety of other cell types in addition to lymphatic endothelium (eg, ICAM-1 and VCAM-1). Most importantly, all of them are also involved in leukocyte extravasation through the vascular endothelium.
Selective expression of endothelial MR on lymphatics and its absence on blood vasculature place MR in a unique position among the homing-associated molecules, most of which operate only in the blood vasculature or are universally involved in cell trafficking.1,2,40 Therefore, while therapeutic intervention aimed at blocking the function of homing-associated molecules such as ICAM-1 and VCAM-1 could potentially affect normal cell trafficking and immune functions, inhibition of MR could selectively reduce leukocyte migration from the periphery into the draining lymph nodes during inappropriate inflammatory reactions. Furthermore, similar strategies could also be effective in reducing metastatic seeding via the lymphatic vasculature, for instance as an adjunctive therapy during cancer surgery.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michel Nussenzweig for donating the MR−/− mice; Tero Vahlberg, Kaisa Auvinen, Suvi Nevalainen, Jouko Sandholm, Maritta Pohjansalo, Sari Mäki, and Laila Reunanen for advice and technical help; Christopher Bayliss for revising the language; and Anne Sovikoski-Georgieva for secretarial help.
This work was supported by funding from the Academy of Finland (Helsinki), the Finnish Cancer Organizations (Helsinki), the Sigrid Juselius Foundation (Helsinki, Finland), the Arvo and Inkeri Suominen Foundation (Salo, Finland), and the Technology Development Center of Finland (Helsinki).
Authorship
Contribution: F.M.-I., R.T., M. Miiluniemi, M.K., M. Maksimow, and J.N. performed the experiments and contributed to the writing of the paper; L.M.-P. provided key reagents and contributed to the writing of the paper; M.S. designed the work and wrote the paper; S.J. performed the experiments, designed the work, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sirpa Jalkanen, MediCity Research Laboratory, Tykistökatu 6A, 20520 Turku, Finland; e-mail:sirjal@utu.fi.
References
Author notes
*R.T. and M. Miiluniemi contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal