In this issue of Blood, Stegenga and colleagues at the University of Amsterdam report an elegant series of studies probing the independent effects of glucose and insulin on endogenous inflammatory and coagulant responses in a well-characterized model of acute inflammation—bolus endotoxin challenge in human volunteers.
Diabetes mellitus affects more than 170 million people worldwide, or 2.8% of the population of the planet; its prevalence is expected to rise to 4.4% by 2030.1 The morbidity of diabetes is linked to a spectrum of procoagulant and inflammatory derangements. Conversely, abnormalities of glucose regulation are common to a number of acute and chronic inflammatory disorders.2 By clamping plasma levels of glucose or insulin at normal or elevated levels, Stegenga and colleagues demonstrate that glucose and insulin differentially regulate neutrophil and coagulant response to endotoxin challenge. Hyperglycemia alone inhibits the release of neutrophil elastase and enhances levels of thrombin:antithrombin (TAT) complexes, whereas hyperinsulinemia reduces plasminogen activator (PA) activity and increases both the level and the activity of plasminogen activator inhibitor type 1 (PAI-1). Neither significantly alter circulating levels of key cytokines whose release is induced by endotoxin, nor do they affect neutrophil numbers or markers of endothelial-cell activation. The effects are specific to the regulation of coagulation, and both promote a net procoagulant state.
These are important studies, but their interpretation is complicated. Endocrine interactions are dynamic: elevated or depressed levels of one hormone evoke a response that modulates levels by altering the release of that hormone, or by inducing the release or inhibition of a counterregulatory hormone. These dynamic interactions were inhibited in this study, both by the clamp technique and by the pretreatment of study subjects with somatostatin to inhibit endogenous insulin release. Nonetheless, 3 key conclusions emerge.
First, hyperglycemia alone supports a procoagulant state by increasing levels of soluble tissue factor and TAT complexes. This observation provides a further rationale for maintaining strict euglycemia in critically ill patients.2
Second, insulin alone, independent of endotoxin, supports a procoagulant state through the induction of PAI antigen and activity, and inhibition of plasminogen activator activity. Thus, exogenous insulin may prove insufficient to reverse the procoagulant state associated with hyperglycemia.
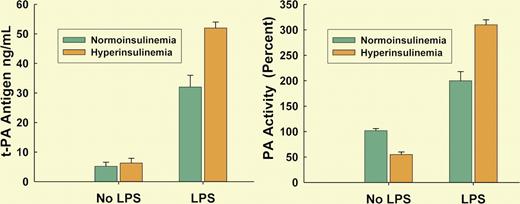
Third, and perhaps most intriguing, is the insight that arises from the characterization of the interaction of an endogenous hormone, insulin, with what might legitimately be considered an exogenous hormone, endotoxin. When data from the current report are compiled with those from the authors' earlier studies in volunteers who had not received endotoxin, it becomes apparent that endotoxin, itself a potent inducer of a procoagulant and proinflammatory state, synergizes with insulin to further increase levels of t-PA antigen and plasminogen activator activity3 (see figure). Endotoxemia is common in critical illness4 in which derangements of glucose-insulin homeostasis are particularly prominent,2 and insights such as those provided by Stegenga et al underline the challenges of modulating the hormonal milieu in a complex and vulnerable patient population.
Circulating levels of t-PA antigen and PA activity in endotoxemic volunteers were both increased by hyperinsulinemia, suggesting that increased insulin primes for an augmented response to endotoxin.
Circulating levels of t-PA antigen and PA activity in endotoxemic volunteers were both increased by hyperinsulinemia, suggesting that increased insulin primes for an augmented response to endotoxin.
Scientific experimentation is inherently reductionist, and imperfect in its capacity to encompass the nuances of diseases that arise within the complexity and intrinsic variability of whole organisms. Nonetheless, thoughtfully applied models such as that reported by Stegenga et al provide the opportunity to tease systematic insights out of the chaos of adaptive biology. And in this initiative, they can offer valuable perspectives on the capacity of living organisms to respond to the vicissitudes of a frequently hostile world, and on the challenges we face in trying to treat the consequences.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal