In this issue of Blood, Ono and colleagues use sophisticated imaging technology to identify a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth.
Platelet adhesion, activation, and aggregation, together with thrombin generation and fibrin formation, are essential steps in the formation of a platelet plug and the arrest of bleeding.1 Important gaps in our knowledge exist regarding the ways by which thrombus size and stability are regulated in flowing blood. Ono and colleagues have used fluorescent markers and an in vitro perfusion system to follow collagen-dependent platelet deposition and fibrin(ogen) incorporation into the building thrombus. Real-time analysis and confocal microscopy reveal a dynamic process with a previously unrecognized contractile activity that assures the tight packing of aggregated platelets. With calcium dependence and kinetics different from those found in fibrin clot retraction, reduced thrombus volume was prevented by blocking nonmuscle myosin heavy chain-IIA (myosin-IIA) activity with blebbistatin and by Rho kinase antagonism. Blocking the tight packing of platelets resulted in the formation of less stable thrombi. Using a novel intravital mouse model for assessing primary hemostasis and FeCl3-induced vascular injury, the investigators confirmed that contractile activity is critical for maintaining the integrity of the hemostatic plug in vivo. Local injection of blebbistatin or a Rho kinase antagonist into the microcirculation resulted not only in a loss of tight packing between platelets in the outer layers of formed thrombi, but also in progressive embolization from the thrombus surface—implying that the process is reversible. Studies on PAR4−/− mice confirmed that thrombin, while necessary for optimal thrombus formation, was not essential for contractile activity. Not only does this study open up new avenues for antithrombotic therapy; it also provides insights into the pathophysiology of MYH9-related platelet disorders that principally affect myosin-IIA.
In an earlier issue of Blood, Chen et al2 examined the role of MYH9 in thrombocytopoiesis, and showed how myosin-IIA complexes and their upstream signaling pathways regulate megakaryocyte migration and the timing of proplatelet formation. They concluded that a lifting of the restraints imposed on proplatelet formation by the Rho-ROCK-MLC-myosin-IIA pathway was a key trigger for proplatelet formation at the sinusoids. A recent study on mice showed that targeted deletion of myosin-IIA in platelets led to a major prolongation of bleeding time and a severe defect in thrombus growth.3 The lack of myosin-IIA led to the absence of shape change and clot retraction in response to platelet agonists, and to platelet morphological changes resembling the human disease. Although MYH9-related human disorders such as May-Hegglin anomaly are autosomal-dominant in inheritance,4 and therefore myosin-IIA deficiency is less extensive,4 altered contractile activity during primary hemostasis may contribute to the bleeding episodes that affect some patients.
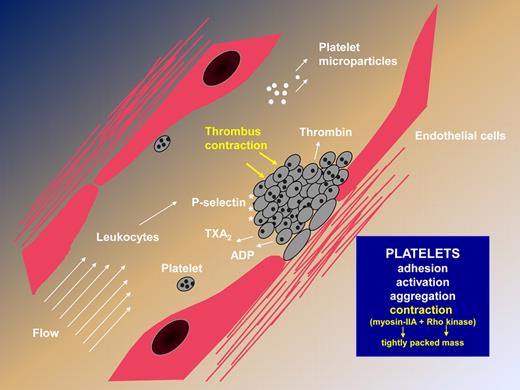
Steps leading to thrombus formation, highlighting the newly described contractile activity that results in a rapidly formed, tightly packed platelet mass independently of fibrin formation.
Steps leading to thrombus formation, highlighting the newly described contractile activity that results in a rapidly formed, tightly packed platelet mass independently of fibrin formation.
The current work by Ono and colleagues in Shaun Jackson's group implies that myosin-IIA complexes play a key role not only in the birth of the platelet, but also in the endgame of hemostatic plug formation and thrombus consolidation in the vessel wall. Because the thrombus is attached to the vessel wall, the contraction will pull the thrombus toward the lesion, simultaneously cementing the breach and facilitating blood flow through the vessel. In the context of atherothrombotic disease, the extent of the contraction may affect the resistance of platelet-rich clots to fibrinolytic agents and to antiplatelet drugs.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal