To the editor:
We read with great interest the papers by Bonig et al1 and Zohren et al,2 which concluded that administration of the monoclonal anti–VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in patients suffering from multiple sclerosis (MS). In these studies, blood samples were serially withdrawn from natalizumab-treated MS patients at different time points after the initiation of natalizumab treatment. Results clearly demonstrated that natalizumab induces a quick and sustained increase of circulating CD34+ hematopoietic progenitors. This is of major interest as, in the context of hematologic malignancies and bone marrow transplantation, natalizumab “might be useful for patients with poor response to granulocyte colony-stimulating (G-CSF)–based protocols.”2 However, we would like to underscore that several important points remain to be addressed with regard to the mobilization of CD34+ progenitor cells in MS patients. First, the actual number and phenotype of blood-circulating CD34+ cells in untreated MS patients needs yet to be accurately assessed. Only a few untreated MS patients were analyzed in both studies (5 in the paper by Zohren et al2 and apparently fewer than 10 in the paper by Bonig et al,1 although this is not clearly stated). Moreover, there is no information on the clinical status of these untreated MS patients (relapsing-remitting form? progressive form? relapse?). Based on the data, one thus cannot conclude that “MS per se is not associated with elevated circulating hematopoietic stem/progenitor cells.”1 In addition, these studies do not provide data on more committed CD34+ cells such as CD34+ myeloid progenitors. This question is of importance because, in the animal model of MS, experimental autoimmune encephalomyelitis (EAE), a mobilization of CD34+ myeloid progenitors was evidenced.3,4 Interestingly, we recently observed that such a mobilization does not occur during the acute phase of the disease (first relapse) but only during the chronic stage of EAE (Figure 1). Once again, this result emphasizes the need for taking into account the clinical status of MS patients when assessing the number of circulating CD34+ hematopoietic stem/progenitor cells (HSPCs). Another important point relates with the fate of CD34+ HSPCs mobilized by natalizumab treatment. Animal studies showed that blood CD34+ hematopoietic stem cells or progenitors target the brain under several pathologic conditions, including EAE.3-5 In this context, CD34+ myeloid progenitors were proposed to provide an expandable source of microglial-like cells in the inflamed brain.3 Surprisingly, the question whether CD34+ HSPCs target the CNS of MS patients is barely discussed in the papers by Zohren et al2 and Bonig et al.1 We feel that at least one important issue should have been mentioned in this regard: the link between CD34+ HPSCs and natalizumab-induced multifocal leukoencephalopathy. Indeed, a small proportion of natalizumab-treated MS patients develop progressive multifocal leukoencephalopathy due to John Cunningham (JC) papovavirus infection.6 Knowing that JC virus can infect hematopoietic progenitor cells,7,8 one may thus consider that mobilizing HSPCs in the blood of MS patients could favor the trafficking of JC virus from the periphery to the CNS.
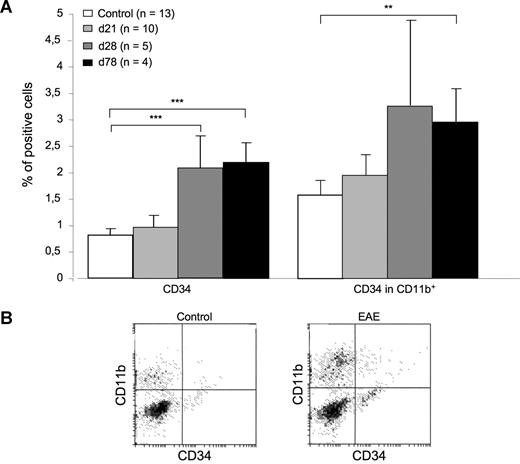
Delayed mobilization of CD34+ myeloid progenitors in EAE mice. (A) The percentage of CD34+ cells was assessed by flow cytometry on peripheral blood mononuclear cells (PBMCs) obtained from control mice or from EAE mice killed on day (d) 21 (first relapse), d28 (chronic phase), or d78 (chronic phase) after immunization (p.i.). The percentage of blood CD34+ cells was not significantly changed in EAE d21 mice versus controls (0.97% vs 0.81%; left panel). However, it significantly increased in EAE d28 mice (2.05%) or EAE d78 mice (2.2%) compared with controls (left panel). Such an increase could be similarly evidenced when considering the percentage of CD34+ cells in the CD11b+ fraction of PBMC (right panel). **P < .01, ***P < .001, Student t test. (B) Data show representative dot plots obtained by FACS analysis of PBMCs obtained from EAE or control mice.
Delayed mobilization of CD34+ myeloid progenitors in EAE mice. (A) The percentage of CD34+ cells was assessed by flow cytometry on peripheral blood mononuclear cells (PBMCs) obtained from control mice or from EAE mice killed on day (d) 21 (first relapse), d28 (chronic phase), or d78 (chronic phase) after immunization (p.i.). The percentage of blood CD34+ cells was not significantly changed in EAE d21 mice versus controls (0.97% vs 0.81%; left panel). However, it significantly increased in EAE d28 mice (2.05%) or EAE d78 mice (2.2%) compared with controls (left panel). Such an increase could be similarly evidenced when considering the percentage of CD34+ cells in the CD11b+ fraction of PBMC (right panel). **P < .01, ***P < .001, Student t test. (B) Data show representative dot plots obtained by FACS analysis of PBMCs obtained from EAE or control mice.
Authorship
Acknowledgment: This work has been supported by a grant from Association pour la Recherche sur la Sclérose en Plaques (ARSEP, Paris, France) to S.N.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Serge Nataf, Inserm U842, Lyon, F-69372, France; e-mail: serge.nataf@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal