Abstract
Stimulation through the B-cell antigen receptor (BCR) is believed to be involved in the natural history of chronic lymphocytic leukemia (CLL). Some cases respond to the in vitro cross-linking of surface immunoglobulin (sIg) with effective activation. In contrast, the remaining cases do not respond to such stimulation, thereby resembling B cells anergized after antigen encounter in vivo. However the biochemical differences between the 2 groups are ill defined, and in humans the term B-cell anergy lacks a molecular definition. We examined the expression and activation of key molecules involved in signaling pathways originating from the BCR, and we report that a proportion of CLL patients (a) expresses constitutively phosphorylated extracellular signal-regulated kinase (ERK)1/2 in the absence of AKT activation; (b) displays constitutive phosphorylation of MEK1/2 and increased nuclear factor of activated T cells (NF-AT) transactivation; and (c) is characterized by cellular unresponsiveness to sIg ligation. This molecular profile recapitulates the signaling pattern of anergic murine B cells. Our data indicate that constitutive activation of mitogen activated protein (MAP) kinase signaling pathway along with NF-AT transactivation in the absence of AKT activation may also represent the molecular signature of anergic human B lymphocytes. CLL cases with this signature may be taken as a human model of anergic B cells aberrantly expanded.
Introduction
Chronic lymphocytic leukemia (CLL) is a chronic lymphoid malignancy characterized by the accumulation of CD5+ monoclonal B lymphocytes in primary and secondary lymphoid tissues. Several lines of evidence, including molecular and functional analysis of the monoclonal immunoglobulin (Ig), strongly support the hypothesis that a stimulation through the B-cell receptor (BCR) may be involved in the selection and expansion of the malignant clone.1,2
At molecular level, the leukemic cells have a preferential IGHV gene usage; in at least half of the cases, they carry somatically mutated IGHV genes3,4 ; in more than 20% of the cases, they express closely homologous if not identical (“stereotyped”) complementarity-determining region 3 (CDR3) sequences on both heavy and light chains.5-11 All these observations suggest that a limited set of antigenic elements may be specifically recognized by the leukemic Ig.
At functional level, it is long known that CLL cells characteristically express low levels of surface Ig (sIg) a property shared by murine B cells anergized by the exposure to antigens (Ags).1,2 This anergy-related feature does not necessarily indicate a functional impairment of BCR signaling, as at least half of the cases can actually be stimulated in vitro through their sIg, irrespective of their low level.12 In contrast, the remaining CLL cases are unresponsive to BCR cross-linking, thereby resembling also at functional level B cells anergized in vivo after chronic stimulation by an antigen.13 Still, and despite extensive studies, the molecular bases for this functional difference are ill defined.
In normal B lymphocytes, BCR engagement triggers a cascade of signaling events resulting in enzymatic activation of various kinases and phosphorylation of the relevant substrates.14 In particular, BCR-proximal Lyn and Syk kinases induce PLCγ2 phosphorylation and Ras activation. Ras binds to and activates Raf kinase that subsequently activates MEK1 and MEK2 that lay immediately upstream of extracellular signal-related kinase (ERK)1/2. In a parallel signaling cascade, PI3K phosphorylates and activates AKT kinase. BCR stimulation activates gene transcription also through activation of nuclear factor (NF)-kB and nuclear factor of activated T cells (NF-AT) transcription factors14-16 (and see the Science STKE website for a schematic representation16 ).
Several groups have examined the signaling pathways originating from the BCR in CLL cells. These studies indicate that some elements are constitutively activated in all CLL patients. Specifically, it has been reported that CLL B cells contain high Lyn tyrosine kinase activity17 and constitutively active p38.18,19 Active nuclear NF-AT and NF-kB transcription factors were also found in unstimulated CLL B cells,20-22 whereas controversial data have been reported for the activation of AKT kinase pathway. One group has reported constitutive activation of PKC and PI3K but not of AKT in CLL cells,18 in contrast to other investigators who described constitutive activation of AKT.23 A recent report24 suggested that BCR downstream signaling pathways are intact in CLL cells as, in a group of patients, the BCR unresponsiveness was reversible in vitro and was dependent on the actual level of sIgM. It has also been proposed that the expression of ZAP-70 may be critical in determining the increased BCR signaling capacity likely through the inhibition of events that terminate the response.25,26
We examined the expression and activation of key molecules involved in signaling pathways originating from the BCR in a group of CLL patients with properly characterized biologic and clinical features. Here we show that the MAPK extracellular signal-regulated kinase (ERK) is constitutively phosphorylated, while AKT is not, in a proportion of patients whose cells are unresponsive to in vitro sIg ligation. These cases also constitutively express phospho-MEK1/2 and show NF-AT transcription factor activity. This molecular profile reproduces the signaling pattern of anergic murine B cells.13,27-29 Therefore, we propose that constitutive activation of MAP kinase signaling pathway in the absence of AKT activation along with NF-AT transactivation also represents the molecular signature of human anergic B lymphocytes. CLL cases presenting this signature may be taken as a human model of anergic B cells aberrantly expanded.
Methods
Antibodies and reagents
Antiphosphotyrosine, anti-Lyn, and anti-Syk antibodies for immunoprecipitation (IP) and Western blot (WB) were purchased from Upstate Biotechnology (Bar Harbor, ME). Anti–β-actin antibody for WB was from Sigma-Aldrich (St Louis, MO). Anti–phospho-ERK, anti–phospho-AKT, anti-AKT, and anti–phospho-MEK for WB were from Cell Signaling Technology (Danvers, MA). Anti-ERK2, anti-MEK, and anti–phospho-IkB antibodies for WB were from Santa Cruz (Santa Cruz, CA). NF-ATc1 activation was analyzed using TransAM transcription factor kit from Active Motif (Carlsbad, CA).
Cell culture and stimuli
MEC1, Daudi, and Jurkat cell lines were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ, Braunschweig, Germany) and cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, and 15 μg/mL gentamicin (complete RPMI; Invitrogen, Carlsbad, CA).
Leukemic cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, and 15 μg/mL gentamicin (complete RPMI; Invitrogen).
Purified leukemic B cells were cultured at a concentration of 3 × 106 cells/mL in the presence or absence of goat F(ab)2 anti–human IgM antibodies (10 μg/mL; CALTAG Laboratories, San Jose, CA) or phorbol 12-myristate 13-acetate (PMA, 100 ng/mL; Sigma-Aldrich) for 3 minutes.
Tissue samples and cell purification
Leukemic lymphocytes were obtained from peripheral blood of CLL patients, diagnosed according to National Cancer Institute Working Group (NCIWG) guidelines.30 All patients were either untreated or off therapy for at least 6 months before the beginning of the study. The following parameters were analyzed for each patient: age, sex, disease stage at diagnosis according to Binet31 or modified Rai32 criteria, CD38 expression, IGHV gene mutational status,33 ZAP-70 expression,34 history of treatment, characterization of disease as progressive or stable as defined by the NCIWG,30 and survival time.
Leukemic CD19+ cells were purified from fresh peripheral blood using a B-lymphocyte enrichment kit (RosetteSep; StemCell Technologies, Vancouver, BC), or after thawing using MACS-microbeads for negative selection35 (Miltenyi Biotec, Auburn, CA). Purity of all preparations was always more than 99%, and the cells coexpressed CD19 and CD5 on their cell surfaces as checked by flow cytometry (FC500; Beckman Coulter, Hialeah, FL); preparations were virtually devoid of natural killer (NK) cells, T lymphocytes, and monocytes.
In selected cases, fresh and frozen samples from the same CLL patients were analyzed in parallel and gave identical results in terms of ERK phosphorylation.
All tissue samples were obtained with approval by the institutional review board of San Raffaele University Hospital (Milan, Italy). Informed consent was obtained in accordance with the Declaration of Helsinki.
Western blot analysis
Cells (10 millions of cells per sample) were pelleted, resuspended in cold lysis buffer (150 mM NaCl, 1% NP40, 50 mM Tris HCl, 1 mM EDTA, protease inhibitors, phosphatase inhibitors) and spun in microcentrifuge at 4°C for 20 minutes at 16 000g; lysate was recovered from supernatant.
Whole protein extracts from either fresh or thawed cells were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and proteins from gel were electron-transferred onto nitrocellulose membranes and incubated for 2 hours with the indicated antibodies. Immunoreactivity was revealed by incubation with either goat anti–rabbit Ig or goat anti–mouse Ig (Upstate Biotechnology) conjugated with HRP, and was followed by enhanced chemiluminescence (ECL) reaction (Pierce, Rockford, IL) and film exposures.
Densitometric analysis of ERK-specific bands was performed using ImageQuant Software (GE Healthcare, Piscataway, NJ). The values of individual patients were calculated as percentage of the positive control (MEC1 cell line) and determined as the ratio of the OD of p-ERK and OD of total ERK. The values of stimulation experiments were calculated as ratio of (OD of stimulated/unstimulated p-ERK) to (OD of stimulated/unstimulated total ERK).
Results
ERK but not AKT is constitutively phosphorylated in a proportion of CLL patients
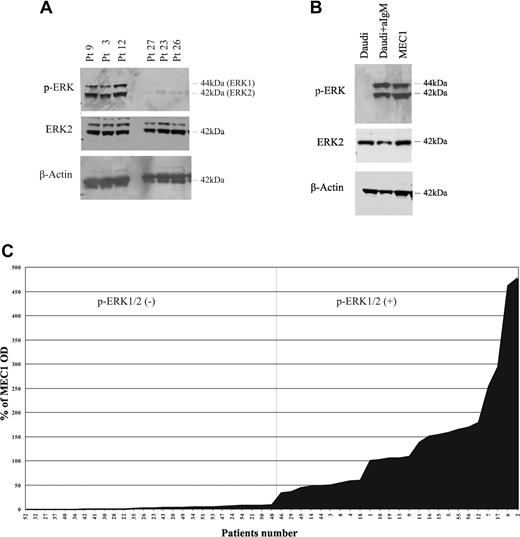
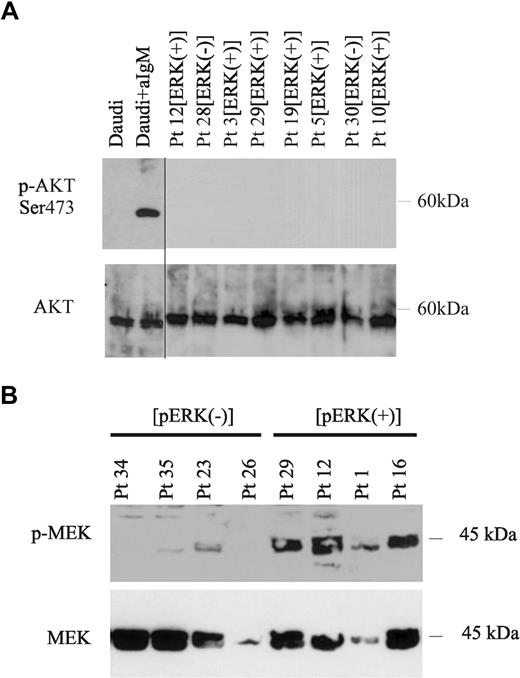
To determine the expression and activation status of BCR-mediated signaling molecules in CLL B cells, we evaluated 2 major signaling pathways activated by BCR that have a critical role in B-cell survival and function, the MEK1/2-ERK1/2 and PI3K/AKT pathways.14-16 Leukemic B lymphocytes were purified from the peripheral blood of 51 CLL patients, and were analyzed for ERK1/2 and AKT phosphorylation by Western blot. Constitutive phosphorylation of ERK1/2 was observed in 25 (49%) of 51 patients (Figure 1A top panel) as revealed by densitometric analysis (Figure 1C) that allowed to clearly distinguish these cases from those who expressed low if any phosphorylation of the protein (26/51 cases, 51%) (Figure 1A,C). MEC1, a CLL cell line,36 also expressed constitutively phosphorylated ERK1/2, while in the human Burkitt lymphoma cell line Daudi, phosphorylation of ERK1/2 was induced only after stimulation with anti-IgM antibodies (Figure 1B top panel).37 Constitutive phosphorylation of ERK in these CLL cases and in MEC1 cells was not influenced by culture conditions, as fetal calf serum deprivation did not alter this expression pattern neither did previous vital freezing procedures (data not shown). In contrast to the constitutive activation of ERK1/2, AKT was not phosphorylated at either serine 473 or at threonine 308, (Figure 2A and data not shown) in any of the 21 CLL samples tested, regardless the phosphorylation status of ERK1/2. We then analyzed several clinical and biologic parameters in all patients (Table 1) studied for ERK phosphorylation status. These parameters included age at diagnosis, sex, disease stage at diagnosis according to Binet31 or modified Rai32 criteria, CD38 expression, IGHV gene mutational status,33 ZAP-70 expression,34 history of treatment, characterization of disease (as progressive or stable as defined by the NCIWG30 ), and survival time.
Phosphorylation of ERK in CLL cells. (A) Extracts from CLL cells purified from peripheral blood of 6 representative patients (of 51 tested) were analyzed for phosphorylation of ERK1/2 by Western blot with p-ERK1/2–specific antibody (top panel). Immunoblot was stripped and reblotted with ERK2-specific antibody (middle panel) or β-actin–specific antibody (bottom panel). (B) Extracts from unstimulated Daudi cells, anti-IgM–treated Daudi cells, and unstimulated MEC1 cells were analyzed by SDS-PAGE followed by immunoblot with p-ERK1/2 antibody (top panel), followed by stripping and reblotting of the immunoblots with ERK2-specific antibody (middle panel) or β-actin–specific antibody (botttom panel). (C) Densitometric analysis of p-ERK/ERK levels in CLL samples. The values of individual patients were calculated as percentage of the positive control (MEC1 cell line) and determined as the ratio of the OD of p-ERK and OD of total ERK.
Phosphorylation of ERK in CLL cells. (A) Extracts from CLL cells purified from peripheral blood of 6 representative patients (of 51 tested) were analyzed for phosphorylation of ERK1/2 by Western blot with p-ERK1/2–specific antibody (top panel). Immunoblot was stripped and reblotted with ERK2-specific antibody (middle panel) or β-actin–specific antibody (bottom panel). (B) Extracts from unstimulated Daudi cells, anti-IgM–treated Daudi cells, and unstimulated MEC1 cells were analyzed by SDS-PAGE followed by immunoblot with p-ERK1/2 antibody (top panel), followed by stripping and reblotting of the immunoblots with ERK2-specific antibody (middle panel) or β-actin–specific antibody (botttom panel). (C) Densitometric analysis of p-ERK/ERK levels in CLL samples. The values of individual patients were calculated as percentage of the positive control (MEC1 cell line) and determined as the ratio of the OD of p-ERK and OD of total ERK.
Constitutive activation of MEK1/2, but not of AKT in p-ERK+ CLL cells. (A) Extracts from Daudi cells either untreated or stimulated with anti-IgM, and from purified CLL cells obtained from the peripheral blood of 8 representative CLL patients previously tested positive for p-ERK1/2 (p-ERK+) or negative for p-ERK1/2 (p-ERK−), were examined for activation of AKT by immunoblot with an antibody specific for phospho-AKT (Ser473). Blots were stripped and reblotted with AKT-specific antibody (bottom panel). A vertical line has been inserted to indicate a repositioned gel lane. (B) Extracts from purified CLL cells isolated from 4 p-ERK1/2− and 4 p-ERK1/2+ CLL patients were examined for MEK phosphorylation by Western blot with an antibody specific for p-MEK1/2 (top panel). Total MEK protein levels were examined on the same blot using an antibody specific for total MEK1/2 (bottom panel).
Constitutive activation of MEK1/2, but not of AKT in p-ERK+ CLL cells. (A) Extracts from Daudi cells either untreated or stimulated with anti-IgM, and from purified CLL cells obtained from the peripheral blood of 8 representative CLL patients previously tested positive for p-ERK1/2 (p-ERK+) or negative for p-ERK1/2 (p-ERK−), were examined for activation of AKT by immunoblot with an antibody specific for phospho-AKT (Ser473). Blots were stripped and reblotted with AKT-specific antibody (bottom panel). A vertical line has been inserted to indicate a repositioned gel lane. (B) Extracts from purified CLL cells isolated from 4 p-ERK1/2− and 4 p-ERK1/2+ CLL patients were examined for MEK phosphorylation by Western blot with an antibody specific for p-MEK1/2 (top panel). Total MEK protein levels were examined on the same blot using an antibody specific for total MEK1/2 (bottom panel).
Clinical and biologic features of the CLL patients studied
| Patient no. . | Age, y . | Sex . | Clinical course . | Stage* . | IGHV,† % . | CD38, % . | ZAP-70 . | Follow-up, mo . | Death‡ . | pERK . |
|---|---|---|---|---|---|---|---|---|---|---|
| 56 | 58 | M | Progressive | 0/A | 97.9 | 40.7 | nd | 144 | no | Pos |
| 29 | 62 | M | Progressive | 0/A | 100 | 81 | Pos | 40 | yes | Pos |
| 46 | 44 | F | Progressive | 0/A | 100 | 4 | Pos | 30 | no | Pos |
| 14 | 74 | M | Stable | 0/A | 87.7 | 3.0 | Pos | 95 | no | Pos |
| 1 | 79 | M | Progressive | 0/A | 100.0 | 41 | Pos | 97 | yes | Pos |
| 4 | 69 | F | Stable | 0/A | 96.7 | 0.7 | Pos | 84 | nd | Pos |
| 3 | 76 | M | Progressive | 0/A | 99.0 | 74 | Neg | 101 | no | Pos |
| 11 | 61 | M | Progressive | 0/A | 89.5 | 1.0 | Pos | 182 | no | Pos |
| 6 | 62 | M | Progressive | 0/A | 94.7 | 0.4 | Neg | 73 | no | Pos |
| 12 | 78 | F | Stable | 0/A | 89.5 | 0.4 | Neg | 101 | no | Pos |
| 10 | 80 | F | Progressive | 0/A | 91.2 | 0.4 | Neg | 222 | no | Pos |
| 5 | 68 | F | Progressive | 0/A | 94.9 | 0.3 | Neg | 91 | no | Pos |
| 9 | 50 | M | Progressive | 0/A | 93.3 | 1.5 | Pos | 105 | no | Pos |
| 13 | 72 | F | Stable | 0/A | 88.4 | 0.1 | Pos | 53 | no | Pos |
| 45 | 83 | F | Progressive | 0/A | 97.6 | 86.0 | Neg | 97 | no | Pos |
| 8 | 68 | M | Progressive | 0/A | 93.5 | 0.4 | Pos | 163 | no | Pos |
| 2 | 76 | F | Progressive | 0/A | 99.0 | 38 | Pos | 54 | no | Pos |
| 7 | 70 | M | Stable | 0/A | 93.7 | 29 | Pos | 49 | no | Pos |
| 15 | 85 | F | Progressive | 1/B | 99.34 | 0.5 | Pos | 44 | no | Pos |
| 16 | 53 | M | Progressive | 1/B | 96.2 | 0.1 | Neg | 159 | no | Pos |
| 44 | 65 | M | Stable | 1/B | 95.4 | 0.1 | nd | 132 | no | Pos |
| 19 | 80 | F | Progressive | 2/B | 98.6 | 28 | Pos | 82 | no | Pos |
| 17 | 74 | M | Progressive | 2/B | 92.5 | 1.0 | Pos | 125 | no | Pos |
| 18 | 53 | M | Progressive | 2/B | 96.2 | 0.1 | Pos | 48 | yes | Pos |
| 55 | 59 | M | Progressive | 4/C | 100.0 | 22.0 | Pos | 63 | yes | Pos |
| 22 | 67 | F | Stable | 0/A | 91.8 | 0.2 | nd | 143 | no | Neg |
| 36 | 59 | F | Progressive | 0/A | 92 | nd | nd | 218 | no | Neg |
| 30 | 79 | M | Stable | 0/A | 92.0 | 0.3 | nd | 216 | no | Neg |
| 34 | 68 | F | Stable | 0/A | 99.31 | nd | nd | 10 | no | Neg |
| 53 | 56 | M | Stable | 0/A | 96.5 | 1.7 | Pos | 60 | no | Neg |
| 20 | 62 | F | Stable | 0/A | 99.7 | 3.7 | Pos | 44 | no | Neg |
| 40 | 75 | M | Stable | 0/A | 94.56 | 0.7 | nd | 16 | no | Neg |
| 21 | 55 | M | Progressive | 0/A | 95.3 | 0.3 | Neg | 107 | no | Neg |
| 37 | 78 | F | Progressive | 0/A | 100 | nd | Pos | 101 | yes | Neg |
| 51 | 58 | M | Stable | 1/B | 87.2 | nd | nd | 30 | no | Neg |
| 48 | 78 | M | Progressive | 1/B | 95.83 | 2.3 | Pos | 76 | no | Neg |
| 54 | 76 | F | Progressive | 1/B | 100 | 15.02 | Pos | 66 | no | Neg |
| 49 | 69 | F | Stable | 1/B | 100.0 | 3.5 | Pos | 6 | no | Neg |
| 42 | 62 | M | Progressive | 1/B | 99.6 | 0.1 | nd | nd | no | Neg |
| 47 | 66 | M | Progressive | 2/B | 91.5 | 5.9 | Neg | 84 | yes | Neg |
| 41 | 47 | F | Progressive | 2/B | 88.9 | nd | nd | 156 | no | Neg |
| 32 | 65 | M | Progressive | 2/B | 95.83 | 0.6 | Pos | 36 | no | Neg |
| 35 | 69 | M | Progressive | 2/B | 95 | 64 | Pos | 120 | yes | Neg |
| 50 | 68 | M | Progressive | 2/B | 100 | 7.4 | Pos | 96 | no | Neg |
| 23 | 82 | M | Progressive | 2/B | 99.0 | 60 | Pos | 162 | no | Neg |
| 24 | 76 | F | Progressive | 2/B | 98.6 | 100 | Pos | 13 | no | Neg |
| 26 | 66 | M | Progressive | 4/C | 100.0 | 99.0 | Pos | 107 | no | Neg |
| 27 | 72 | M | Progressive | 4/C | 96.6 | 16.0 | Neg | 81 | no | Neg |
| 52 | 66 | M | Progressive | nd | nd | 0.1 | Pos | nd | no | Neg |
| 43 | 69 | M | Progressive | nd | nd | 0.9 | Pos | 42 | no | Neg |
| 28 | 57 | M | nd | nd | 91.9 | nd | nd | nd | nd | Neg |
| Patient no. . | Age, y . | Sex . | Clinical course . | Stage* . | IGHV,† % . | CD38, % . | ZAP-70 . | Follow-up, mo . | Death‡ . | pERK . |
|---|---|---|---|---|---|---|---|---|---|---|
| 56 | 58 | M | Progressive | 0/A | 97.9 | 40.7 | nd | 144 | no | Pos |
| 29 | 62 | M | Progressive | 0/A | 100 | 81 | Pos | 40 | yes | Pos |
| 46 | 44 | F | Progressive | 0/A | 100 | 4 | Pos | 30 | no | Pos |
| 14 | 74 | M | Stable | 0/A | 87.7 | 3.0 | Pos | 95 | no | Pos |
| 1 | 79 | M | Progressive | 0/A | 100.0 | 41 | Pos | 97 | yes | Pos |
| 4 | 69 | F | Stable | 0/A | 96.7 | 0.7 | Pos | 84 | nd | Pos |
| 3 | 76 | M | Progressive | 0/A | 99.0 | 74 | Neg | 101 | no | Pos |
| 11 | 61 | M | Progressive | 0/A | 89.5 | 1.0 | Pos | 182 | no | Pos |
| 6 | 62 | M | Progressive | 0/A | 94.7 | 0.4 | Neg | 73 | no | Pos |
| 12 | 78 | F | Stable | 0/A | 89.5 | 0.4 | Neg | 101 | no | Pos |
| 10 | 80 | F | Progressive | 0/A | 91.2 | 0.4 | Neg | 222 | no | Pos |
| 5 | 68 | F | Progressive | 0/A | 94.9 | 0.3 | Neg | 91 | no | Pos |
| 9 | 50 | M | Progressive | 0/A | 93.3 | 1.5 | Pos | 105 | no | Pos |
| 13 | 72 | F | Stable | 0/A | 88.4 | 0.1 | Pos | 53 | no | Pos |
| 45 | 83 | F | Progressive | 0/A | 97.6 | 86.0 | Neg | 97 | no | Pos |
| 8 | 68 | M | Progressive | 0/A | 93.5 | 0.4 | Pos | 163 | no | Pos |
| 2 | 76 | F | Progressive | 0/A | 99.0 | 38 | Pos | 54 | no | Pos |
| 7 | 70 | M | Stable | 0/A | 93.7 | 29 | Pos | 49 | no | Pos |
| 15 | 85 | F | Progressive | 1/B | 99.34 | 0.5 | Pos | 44 | no | Pos |
| 16 | 53 | M | Progressive | 1/B | 96.2 | 0.1 | Neg | 159 | no | Pos |
| 44 | 65 | M | Stable | 1/B | 95.4 | 0.1 | nd | 132 | no | Pos |
| 19 | 80 | F | Progressive | 2/B | 98.6 | 28 | Pos | 82 | no | Pos |
| 17 | 74 | M | Progressive | 2/B | 92.5 | 1.0 | Pos | 125 | no | Pos |
| 18 | 53 | M | Progressive | 2/B | 96.2 | 0.1 | Pos | 48 | yes | Pos |
| 55 | 59 | M | Progressive | 4/C | 100.0 | 22.0 | Pos | 63 | yes | Pos |
| 22 | 67 | F | Stable | 0/A | 91.8 | 0.2 | nd | 143 | no | Neg |
| 36 | 59 | F | Progressive | 0/A | 92 | nd | nd | 218 | no | Neg |
| 30 | 79 | M | Stable | 0/A | 92.0 | 0.3 | nd | 216 | no | Neg |
| 34 | 68 | F | Stable | 0/A | 99.31 | nd | nd | 10 | no | Neg |
| 53 | 56 | M | Stable | 0/A | 96.5 | 1.7 | Pos | 60 | no | Neg |
| 20 | 62 | F | Stable | 0/A | 99.7 | 3.7 | Pos | 44 | no | Neg |
| 40 | 75 | M | Stable | 0/A | 94.56 | 0.7 | nd | 16 | no | Neg |
| 21 | 55 | M | Progressive | 0/A | 95.3 | 0.3 | Neg | 107 | no | Neg |
| 37 | 78 | F | Progressive | 0/A | 100 | nd | Pos | 101 | yes | Neg |
| 51 | 58 | M | Stable | 1/B | 87.2 | nd | nd | 30 | no | Neg |
| 48 | 78 | M | Progressive | 1/B | 95.83 | 2.3 | Pos | 76 | no | Neg |
| 54 | 76 | F | Progressive | 1/B | 100 | 15.02 | Pos | 66 | no | Neg |
| 49 | 69 | F | Stable | 1/B | 100.0 | 3.5 | Pos | 6 | no | Neg |
| 42 | 62 | M | Progressive | 1/B | 99.6 | 0.1 | nd | nd | no | Neg |
| 47 | 66 | M | Progressive | 2/B | 91.5 | 5.9 | Neg | 84 | yes | Neg |
| 41 | 47 | F | Progressive | 2/B | 88.9 | nd | nd | 156 | no | Neg |
| 32 | 65 | M | Progressive | 2/B | 95.83 | 0.6 | Pos | 36 | no | Neg |
| 35 | 69 | M | Progressive | 2/B | 95 | 64 | Pos | 120 | yes | Neg |
| 50 | 68 | M | Progressive | 2/B | 100 | 7.4 | Pos | 96 | no | Neg |
| 23 | 82 | M | Progressive | 2/B | 99.0 | 60 | Pos | 162 | no | Neg |
| 24 | 76 | F | Progressive | 2/B | 98.6 | 100 | Pos | 13 | no | Neg |
| 26 | 66 | M | Progressive | 4/C | 100.0 | 99.0 | Pos | 107 | no | Neg |
| 27 | 72 | M | Progressive | 4/C | 96.6 | 16.0 | Neg | 81 | no | Neg |
| 52 | 66 | M | Progressive | nd | nd | 0.1 | Pos | nd | no | Neg |
| 43 | 69 | M | Progressive | nd | nd | 0.9 | Pos | 42 | no | Neg |
| 28 | 57 | M | nd | nd | 91.9 | nd | nd | nd | nd | Neg |
pos indicates positive as determined by immunoblot analysis; neg, negative as determined by immunoblot analysis; and nd, not determined.
Shown as percentage of similarity to the closest germ line gene.
Resulting from CLL-related events.
The presence of constitutive ERK1/2 phosphorylation significantly correlated with the disease stage at diagnosis according to modified Rai criteria32 (P = .022 using a χ2 test). In particular, 18 (72.0%) of 25 CLL patients carrying phospho-ERK–positive leukemic cells had low stage disease, while only 9 (39.1%) of 23 p-ERK–negative patients had early-stage leukemia. No correlation with other clinical and biologic parameters could be found in this small series.
Constitutive activation of MAPK signaling pathway in phospho-ERK–positive CLL cells
Given the constitutive ERK activation in a proportion of CLL patients, we aimed to determine the status of upstream activators of ERK. We used fresh CLL cells from patients, and we analyzed the phosphorylation status of the proteins involved in the MAPK cascade.
MEK1 and MEK2 kinase (MAP kinase kinases) are phosphorylated after BCR engagement on two serine residues at positions 217 and 221 by Raf. MEK then activates p44 and p42 ERK1/2 MAP kinases by phosphorylating both threonine and tyrosine residues at sites within the activation loop. Using a phospho-MEK1/2 (Ser217/221)–specific antibody, we studied 11 CLL patients and detected constitutively phosphorylated MEK protein only in the cases expressing phospho-ERK (6/11; Figure 2B top panel and data not shown). MEC1 cells also showed constitutively phosphorylated MEK, while similar levels of MEK phosphorylation were detected in Daudi only after activation with anti-IgM antibodies (data not shown).
Constitutive activation of BCR-proximal signaling molecules in CLL cells
Syk and Lyn, the most proximal kinases to the BCR signaling complex, are activated and recruited to the receptor after antigen recognition and subsequently mediate their enzymatic effects on several molecules including PLCγ.14-16
Because it has been reported that CLL B cells carry constitutively phosphorylated Syk and contain high Lyn tyrosine kinase activity,17 which are both upstream from the MAPK pathway, we aimed at determining the activation status of these 2 kinases in the CLL cells from our patients with a different phosphorylation status of MEK/ERK1/2. Consistent with previous observations in CLL,12 we confirmed that all CLL patients tested showed constitutively phosphorylated Syk and Lyn (6 and 4 cases, respectively, data not shown). Extending those observations, our present data show that the constitutive phosphorylation of both molecules in CLL occurs regardless of the ERK1/2 activation status, because it was detected both in p-ERK1/2–positive and p-ERK–negative CLL cells.
Constitutive activation of NF-AT in phospho-ERK–positive CLL cells
We further investigated the expression and activation status of downstream transcription factors that are activated after BCR triggering.
NF-AT family includes NF-AT1 (also called NFATc2 and NFATp) and NF-AT2 (also called NFATc1 and NFATc) transcription factors, both involved in BCR-induced signaling in lymphocytes.14-16 Therefore, we examined the status of NF-AT transcriptional activity in CLL cells with or without constitutive MEK/ERK activation. Using a specific NF-ATc1 transcription factor activity assay, we found that p-ERK–positive CLL cells from 5 of 6 patients showed constitutive NF-AT DNA-binding activity that could be specifically inhibited by a competing oligo nucleotide (Figure 3). In clear contrast, 6 of 6 p-ERK–negative CLL patients showed a significantly lower level of NF-AT DNA-binding activity (Figure 3). MEC1 and Daudi cell lines were used as positive and negative controls, respectively.
Constitutive activation of NF-AT in p-ERK1/2–positive CLL cells. Nuclear extracts of purified CLL cells from 6 p-ERK+ and 6 p-ERK− CLL patients, of Daudi (p-ERK−) and MEC1 cells (p-ERK+) were examined for NF-ATc activation by an enzyme-linked immunosorbent assay (ELISA)–based assay as described in “Antibodies and reagents.” A specific competing oligonucleotide was added to determine specificity of binding. Error bars represent SD.
Constitutive activation of NF-AT in p-ERK1/2–positive CLL cells. Nuclear extracts of purified CLL cells from 6 p-ERK+ and 6 p-ERK− CLL patients, of Daudi (p-ERK−) and MEC1 cells (p-ERK+) were examined for NF-ATc activation by an enzyme-linked immunosorbent assay (ELISA)–based assay as described in “Antibodies and reagents.” A specific competing oligonucleotide was added to determine specificity of binding. Error bars represent SD.
In contrast to NF-AT, which was activated only in p-ERK1/2–positive CLL cells, we observed a constitutive phosphorylation of IkB alpha protein in virtually all (11/12) CLL patients (data not shown). Thus, as previously reported,21,22 NF-kB is constitutively activated in CLL cells and we observed that this event appears to be independent of the phosphorylation status of ERK.
Attenuated BCR signaling in phospho-ERK–positive CLL cells
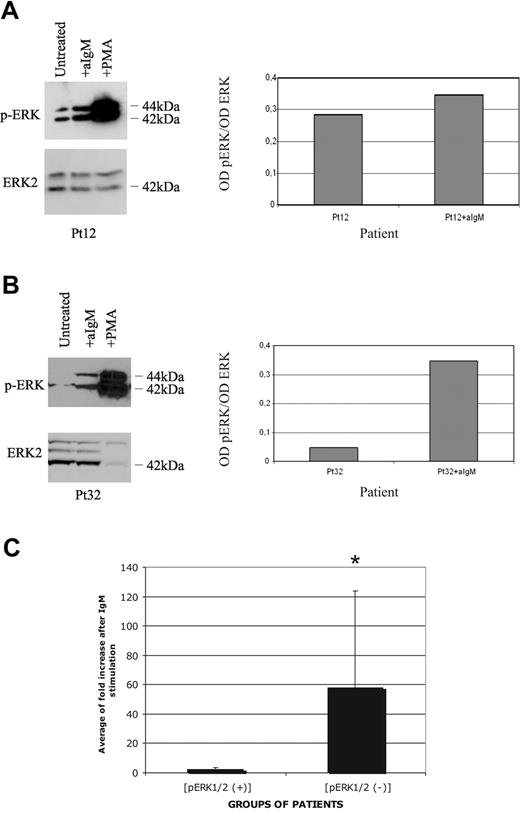
Next we investigated whether the response to Ig stimulation could correlate with ERK phosphorylation status and whether BCR engagement could further increase ERK phosphorylation in those CLL cases that exhibited constitutive ERK activation. Phospho-ERK–positive CLL cells from 5 patients (Figure 4A) showed low or no significant enhancement of the ERK phosphorylation level after stimulation with anti-IgM antibody. A densitometric analysis of the specific bands revealed a mean fold increase of 2.28 (range: 1.25-4.33) compared with the constitutive basal level (Figure 4C). In contrast, in CLL cells from 4 patients who did not display constitutive activation of ERK, sIgM-mediated stimulation in vitro induced invariably strong phosphorylation of ERK (Figure 4B,C). The densitometric analysis showed a median fold increase of 58 (range: 7.0-153.0), compared with initial levels (Figure 4C). Strikingly, PMA that bypasses BCR-proximal events was able to induce phosphorylation of ERK in all CLL cases regardless of the basal phosphorylation status of ERK (Figure 4A,B). Taken together, these results indicate that in p-ERK–positive CLL cases the MAPK signaling machinery is intact but is selectively unresponsive to antigen receptor–mediated activation.
Attenuated BCR signaling in p-ERK–positive CLL cells. (A) One phospho-ERK–positive patient was either untreated, or stimulated with anti-IgM or PMA, and analyzed for ERK1/2 phosphorylation (top panels), or total ERK2 protein levels (bottom panels) by Western blot analysis. Densitometric analysis is reported in the right part of the panel. (B) CLL cells from one phospho-ERK–negative patient were either untreated, or stimulated with anti-IgM or PMA, and analyzed for ERK1/2 phosphorylation (top panels), or total ERK2 protein levels (bottom panels) by Western blot analysis. Densitometric analysis is reported in the right part of the panel. (C) Densitometric analysis of 5 p-ERK+ and 4 p-ERK− patients before and after anti-IgM treatment. Data are expressed as average of fold induction and SD. t test was performed between p-ERK+ and p-ERK− samples; *P < .05.
Attenuated BCR signaling in p-ERK–positive CLL cells. (A) One phospho-ERK–positive patient was either untreated, or stimulated with anti-IgM or PMA, and analyzed for ERK1/2 phosphorylation (top panels), or total ERK2 protein levels (bottom panels) by Western blot analysis. Densitometric analysis is reported in the right part of the panel. (B) CLL cells from one phospho-ERK–negative patient were either untreated, or stimulated with anti-IgM or PMA, and analyzed for ERK1/2 phosphorylation (top panels), or total ERK2 protein levels (bottom panels) by Western blot analysis. Densitometric analysis is reported in the right part of the panel. (C) Densitometric analysis of 5 p-ERK+ and 4 p-ERK− patients before and after anti-IgM treatment. Data are expressed as average of fold induction and SD. t test was performed between p-ERK+ and p-ERK− samples; *P < .05.
Discussion
Several pieces of evidences indicate that stimulation through the BCR is a central event in the natural history of CLL.1 CLL cells are nevertheless heterogeneous in terms of their ability to respond to stimulation via the antigen receptor.12,38,39 At least half of the cases can actually be stimulated in vitro through their sIg. In contrast, the remaining CLL cases are unresponsive to BCR cross-linking, as determined by the absence of tyrosine phosphorylation,12 thereby recalling B cells anergized in vivo after chronic stimulation by an antigen.40 Anergy is one of the physiological mechanisms necessary to silence autoreactive B cells to reach tolerance13 and has been studied mainly in murine models, taking advantage of double transgenic mice expressing single-idiotype immunoglobulin and its cognate antigen. These studies identified some functional features restricted to anergic B cells such as attenuated BCR signal transduction, reduced sIgM expression, and limited lifespan. At molecular level, anergic murine B lymphocytes have a constitutive expression of tyrosine phosphorylation and show activated ERK that cannot be further induced after BCR stimulation. NF-AT exhibits nuclear localization and is constitutively activated in these anergic mouse B cells.27,29 Interestingly, peritoneal CD5+ B1 cells that fail to be fully activated by BCR stimulation and to induce other BCR-induced signaling pathways also show similar biochemical features; as an example, phosphorylation of AKT-Ser473 is neither present nor can be induced after in vitro stimulation of surface Igs.28 While these findings establish a distinct signature of B-cell anergy in the murine system,41 in the human immune system the term B-cell anergy has been used primarily to describe the overall failure to respond to stimulation through the Ig receptor and lacks a molecular definition.
Here, we show that the MAPK ERK is constitutively phosphorylated in a proportion of CLL patients, characterized by unresponsiveness to BCR triggering in vitro. These cases also constitutively express phospho-MEK1/2 and show NF-AT transcription factor activity. Interestingly, these molecular features are not associated with AKT phosphorylation, which would be expected when a B lymphocyte is fully activated by the antigen encounter. Interestingly, the concomitant presence of all these molecular features recapitulates the signature reported in the murine system.27,29,42,43 Because it is also associated with attenuated response to BCR engagement, it may be regarded as a molecular imprint of anergization in human B cells as well. The “molecularly anergic” subset of CLL described in this study may represent a cellular model of anergic human B cells aberrantly expanded as part of the malignant process.
It is puzzling that MEC1 cell, a CLL cell line, also shows similar features (constitutive activation of ERK1/2, MEK, NF-AT) in clear contrast to a lymphoma cell line like Daudi. Although it is conceivable that these features might be ascribed to several other reasons including additional genetic defects, it is also possible to speculate that the “anergic” phenotype might resemble the in vivo anergic state of the patient's cell from which the cell line derived and that was somehow frozen in a particular functional state due to the in vitro transformation. Further studies on MEC1 as a potential model of anergy may be warranted and may help to clarify these issues.
Continuous binding of antigen and subsequent receptor signaling are essential for the maintenance of anergy in B lymphocytes.42 This is in accordance with a recent report showing that IgM unresponsiveness in CLL cells can be restored following in vitro culture, suggesting the possibility of a direct and sustained engagement of the receptor by a putative antigen in vivo.24 Sustained antigen receptor engagement would result in the presence of an active signaling cascade, consistent with our present observations of constitutive in vivo activation of the MAP kinase pathway in a distinct proportion of CLL patients. Although in the present study we focused on the analysis of the BCR-regulated signaling pathways, it should be kept in mind that activation of MAPK may be induced by the ligation of other surface receptors, including receptors for growth factors, cytokines, and chemokines.44 Further studies are required to address the role of such signals in CLL cells.
In mouse models, the anergic B cells present within a normal repertoire were found to be enriched for autoreactive B cells.27 This finding suggests that anergy is probably the primary mechanism through which autoreactive B cells are silenced and may be critical for prevention of autoimmunity. Interestingly, recent observations indicate that CLL cases, especially those carrying stereotyped Ig receptors, express highly polyreactive and autoreactive antibodies.8,45 In light of those observations, our present data lead to the intriguing hypothesis that phospho-ERK–positive CLL cells might derive from the leukemic transformation of previously anergized autoreactive B lymphocytes. It will be challenging to investigate whether specific subsets of CLL patients characterized by the expression of stereotyped receptors7,8,46 are enriched for the presence of constitutively phosphorylated ERK and the absence of AKT activation.
Constitutive ERK phosphorylation has been reported in a number of other lymphoid malignancies including hairy cell leukemia (HCL)47 and adult lymphoblastic leukemia (ALL).48 Constitutive phosphorylation of ELK (a direct substrate of ERK) and p38 has been reported in a variety of B-cell lymphoproliferative disorders including 2 cases of CLL.49 To what extent the molecular signature of anergy observed in our present studies relates to the CLL clinical outcome is still undefined. ERK phosphorylation seems to define CLL cases with a favorable presentation at diagnosis, which is not surprising as Ig-unresponsive CLLs are known to be enriched for cases with a better clinical outcome.12 The reason why we were unable to find a correlation between constitutive ERK phosphorylation and other biologic prognostic factors including IGHV gene mutational status might be related to the relatively low number of cases studied. It will also be interesting to analyze whether the constitutive activation of these signaling molecules is a stable feature or changes during the course of the disease, and whether it correlates with changes in the clinical status.
Finally, our data suggest that MAPK pathway may be regarded as a potential target for pharmacological intervention in the selected cohort of phospho-ERK–positive patients. This could be achieved by exploiting farnesyltransferase inhibitors, a class of target-specific anticancer agents that block Ras activity and downstream signaling pathways including ERK phosphorylation.50 These compounds have already been shown to induce apoptosis in CLL cells,51 although their molecular mechanism of action is currently ill defined. It would be interesting to investigated whether responsiveness to farnestylation inhibitors is preferentially observed in CLL cases with constitutive ERK phosphorylation. Pharmacological inhibition of the MAPK pathway has also been shown to increase sensitivity to purine analogues in CLL cells,52 suggesting that combined modality treatment in selected patients may increase the curative index.
In conclusion, our results couple BCR unresponsiveness to a constitutive activation pattern of the MAP kinase pathway in a subset of CLL patients. These features might be considered a molecular signature of in vivo cellular anergization and suggest a potential mechanism responsible for the distinct biologic behavior of the leukemic cells and the indolent clinical course in a subset of CLL patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sabrina Bertilaccio, Antonis Dagklis, and Claudia Fazi for helpful and collaborative support in the laboratory.
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano), CLL Global Research Foundation, Progetti di Ricerca di Interesse Nazionale (PRIN), Fondo per gli Investimenti della Ricerca di Base (FIRB), Progetto Integrato Oncologia (PIO), Italian Ministry of Foreign Affaire, and Fondazione Ferrero. C.S. is supported by Fondazione Anna Villa e Felice Rusconi (Varese, Italy). B.A. is supported by Sovvenzione Globale INGENIO.
Authorship
Contribution: M.M. designed research, performed experimental work, analyzed and interpreted data, and drafted the paper; B.A., C.S., and M.F. performed experimental work, and analyzed and interpreted data; I.V. collected clinical data and performed statistical analysis; V.B. analyzed data and edited the paper; P.G. designed research, analyzed data, and wrote and edited the paper; F.C.-C. designed and supervised research, analyzed and interpreted data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Federico Caligaris-Cappio, Università Vita-Salute San Raffaele, Via Olgettina 60, 20132, Milano, Italy; e-mail: caligaris.federico@hsr.it.