Abstract
During thymocyte development, the T-cell receptor (TCR) can discriminate major histocompatibility complex (MHC)/peptide ligands over a narrow range of affinities and translate subtle differences into functional fate decisions. How small differences in TCR input are translated into absolute differences in functional output is unclear. We examined the effects of galectin-1 ablation in the context of class-I–restricted thymocyte development. Galectin-1 expression opposed TCR partial agonist-driven positive selection, but promoted TCR agonist-driven negative selection of conventional CD8+ T cells. Galectin-1 expression also promoted TCR agonist-driven CD8αα intestinal intraepithelial lymphocytes (IEL) development. Recombinant galectin-1 enhanced TCR binding to agonist/MHC complexes and promoted a negative-selection-signaling signature, reflected in intensified rapid and transient extracellular signal-regulated kinase (ERK) activation. In contrast, galectin-1 expression antagonized ERK activity in thymocytes undergoing positive selection. We propose that galectin-1 aids in discriminating TCR-directed fate decisions by promoting TCR binding to agonist/MHC complexes and enforcing agonist-driven signals, while opposing partial-agonist signals. In this way, galectin-1 widens the distinction between TCR-directed functional fate cues.
Introduction
During T-cell development in the thymus, thymocytes are subjected to selection processes designed to ensure the generation of a diverse repertoire of functional T cells (positive selection), the removal of self-reactive thymocytes with auto-aggressive potential (negative selection), and the development of regulatory T populations that function in maintaining self-tolerance.1 Selection is cued through T-cell receptor (TCR) interactions with specific self-peptide/major histocompatibility complexes (MHCs) expressed by thymic antigen-presenting cells. Very weak TCR-peptide-MHC interactions are insufficient to elicit signals required for thymocyte survival, whereas exceptionally strong TCR-agonist peptide-MHC interactions direct thymocyte apoptosis during negative selection or promote the generation of regulatory CD8αα intestinal intraepithelial lymphocytes (IEL) or T-regulatory cells.2-4 Thymocytes bearing TCRs with intermediate affinity for self-peptide/MHC complexes (partial agonists) are cued to survive and initiate programs for development into mature T-helper or cytotoxic T lymphocyte (CTL) lineages during positive selection.4 How the TCR discriminates subtle differences in ligand binding and translates them into distinct signals and functional fates remains unresolved. Recent findings indicate that developing thymocytes convert small differences in TCR binding affinity into discrete functional outcomes by controlling the compartmentalization, duration, and intensity of mitogen-activated protein (MAP) kinase signaling cascades.5,6 However, it remains unclear how TCR engagement differentially couples to MAP kinase activation pathways under these circumstances
Immunologists have long recognized that developing thymocytes express characteristic patterns of cell surface glycosylation.7,8 Plant lectins were first used to define thymocyte subsets expressing particular oligosaccharide ligands.7,8 For instance, peanut agglutinin (PNA) binds developing CD4+CD8+ double-positive (DP) thymocytes, but not mature CD4+ or CD8+ single-positive (SP) thymocytes due to its specificity for the O-linked disaccharide Galβ1,3GalNAc, which becomes masked on mature SP thymocytes due to sialic acid addition.8 In contrast, Sambucus nigra lectin (SNA), a lectin that recognizes α-2, 6-linked sialic acid on N-glycans, binds SP thymocytes, but not DP thymocytes.7,9 The identification of endogenous lectins expressed in the thymus suggests they may similarly function in discriminating thymocyte subsets and may contribute to the regulation of thymocyte development.10
One such lectin, galectin-1 (gal-1), is a member of a family of endogenous β-galactose–binding proteins.10,11 Gal-1 is secreted as a monomer that can form a noncovalently-linked homodimer with 2 carbohydrate recognition domains. Gal-1 has minimal specificity for N-acetyllactosamine,12 but it preferentially binds glycans bearing repeating units of N-acetyllactosamine.12,13 Despite abundance of such structures, gal-1 binds a discrete subset of T-cell surface glycoproteins, presumably because they decorate their protein backbones with repeating lactosamine units spaced in a way that favors multivalent and high avidity gal-1 binding and packing. This feature likely accounts for the ability of gal-1 to form and segregate glycolattice-based microdomains composed of discrete gal-1 counterligands.14,15 Indeed, exposure of recombinant galectin-1 (rgal-1) to thymocytes led to the formation of segregated microdomains composed of CD45/CD3 and CD43/CD7.14 In addition, on subsets of developing and activated T cells, gal-1 preferentially binds properly glycosylated CD3, CD4, CD7, CD43, and CD45, each of which are known to contribute to TCR signal transduction and thymocyte development.14,16,17 In the thymus, gal-1 binds both CD3lo and CD3hi thymocytes, though it preferentially binds to the immature CD3lo subpopulation subject to thymocyte selection processes.10
Previously, we identified gal-1 as a novel T-cell regulator, capable of tuning TCR signals to selectively modulate functional outcome,18,19 and demonstrated that rgal-1 cooperates with anti-TCR engagement to induce apoptosis in DP thymocytes.19 Together with other studies,20,21 these findings led us to predict that endogenous gal-1 might set TCR signaling thresholds during T-cell development.
To test this hypothesis, we completely backcrossed mice with the gal-1 gene disrupted onto the C57Bl/6 and TCR transgenic backgrounds and tracked thymocyte development into mature conventional CD8+ T cells and CD8αα IEL. We found that gal-1 expression enhanced negative selection of CD8+ T cells and promoted development of gut CD8αα IEL, selection events which rely on TCR engagement with agonist ligand.3 Treatment of DP thymocytes with rgal-1 increased binding avidity of the TCR to agonist peptide/MHC Kb tetramers. Further, addition of rgal-1 to in vitro negative selection models promoted apoptosis and intensified the transient ERK signaling signature associated with negative selection. Conversely, gal-1 expression selectively inhibited partial agonist-driven positive selection of conventional CD8+ T cells and antagonized ERK activity in thymocytes undergoing positive selection. These data identify gal-1 as a selective modifier of TCR-driven functional fate during thymocyte development and provide evidence that it acts by differentially modulating activation of TCR-induced MAP kinase signals. These findings lend insight into mechanisms of TCR signal specificity control during thymocyte development and elucidate a role for glycosylation in directing thymocyte developmental fate.
Methods
Mice
Mice (129 S/V gal-1−/−)22 were backcrossed 13 generations with C57Bl/6 mice to produce gal-1−/− C57Bl/6 mice. Gal-1−/− H-Y Rag2−/− and gal-1−/− OT-1 Rag2−/− mice were generated by crossing gal-1−/− mice to H-Y Rag2−/− or OT-1 Rag2−/− mice, respectively. Experiments followed an approved protocol of the University of California Los Angeles Chancellor's Animal Research Committee. Mice were genotyped using the following: H-Y 5′-GACATTGAGCTGTAATCAGAC, 3′-ACAGCGTTTCTGCACTGTTATCACC; OT-1 5′-aaggtggagagagacaaagga, 3′-ccagtgcatgcatacctcag; gal-1+5′-tggtggagcaggtctc-agga, 3′-tgagacattccccaggtttga; gal-1-5′-ccttgcaaag-tccagtattctgc, 3′-acctgcgtgcaatccatcttg; Rag2+5′-gggaggacactcacttgccagta; Rag2-5′-cggccggagaa-cctgcgtgcaa; Rag2±3′-agtcaggagtctccatctcactga. Polymerase chain reaction (PCR) conditions were 94°C for 3 minutes, 94°C for 45 seconds, 60°C for 45 seconds, 72°C for 1 minute 30 seconds (40 cycles), and 72°C for 10 minutes.
Immunohistochemistry
Organs were fixed in 10% formalin. Sections were blocked in 3% H2O2, followed by 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS), incubated with polyclonal rabbit anti–gal-1 (Strategic Biosolutions, Newark, CA) or preimmune serum overnight at 4°C, followed by goat anti–rabbit horseradish peroxidase (HRP; Biorad, Hercules, CA) at room temperature, developed with 3-amino-9-ethylcarbazole (AEC; Biomeda, Foster City, CA) at 37°C, counterstained with hematoxylin, and mounted with glycerol-gelatin (Sigma-Aldrich, St Louis, MO). Images (10×) were captured at room temperature using 14.2× color mosaic camera (Diagnostic Instruments, Sterling Heights, MI) mounted to an Olympus BX50 microscope and processed with Spot software, v4.6 (Diagnostic Instruments).
Thymocyte and splenocyte staining
Thymocytes and splenocytes from mice (6.2 weeks) were counted and stained with anti-CD3, -CD4, -CD8, and -CD24 (BD Biosciences, La Jolla, CA). Thymocytes from mice (8 weeks) were also stained with biotinylated SNA or PNA (Vector Laboratories, Burlingame, CA) and streptavidin Tricolor (Caltag, Carlsbad, CA). Samples were acquired on a FACSCalibur (BD Biosciences) and analyzed with Cellquest (BD Biosciences).
Fetal thymic organ cultures
Fetal thymi were obtained from fetuses of day-14 pregnant C57Bl/6 females (The Jackson Laboratories, Bar Harbor, ME) and cultured on styrofoam rafts with L2hmda for 7 days. L2hmda is a low-molecular-weight, highly hydrophilic, doubly charged, divalent β-galactoside derivative, which binds gal-1 approximately 100 times stronger than lactose or lactosamine.23,24 Day-21 thymocytes were stained with anti-CD4 and anti-CD8.
CD8+ T-cell stimulation
CD8+ T cells were obtained using the CD8+ T-cell isolation kit (Miltenyi, Auburn, CA) and magnetic sorting (Miltenyi). Cells were stimulated in 6-well plates coated with anti-CD3 (2 μg/mL)/anti-CD28 (2 μg/mL) for 48 hours, and stained with anti-CD8, -Vα2, and -CD25.
IEL preparation and staining
Small intestines were dissected from mice, cut longitudinally, and into 1-cm segments. Segments were shaken in Hanks balanced salt solution (1 mM dithiothreitol, 15% fetal calf serum) at 37°C for 40 minutes and separated from solution. IEL were obtained at interface of the 40%/70% Percoll gradient and stained with anti-Vβ8.1-8.2 or -Vα2, -CD8α, -CD8β, and -CD103 antibodies.
pERK staining
Staining was performed as described by Krutzik et al.25 For OT-1 thymocytes, cells were immediately fixed after isolation in formaldehyde for 10 minutes at room temperature, permeabilized with ice-cold methanol for 30 minutes, washed, and stained with anti-CD4, -CD8, and pERK Alexafluor 647 (Cell Signaling Technologies, Danvers, MA) for 30 minutes at room temperature. Anti-pERK recognizes pERK1/pERK2; staining was abrogated by MAPK kinase (MEK) inhibitor, U0126 (data not shown). For C57Bl/6 thymocyte stimulation, 106 gal-1−/− thymocytes were stimulated in 12-well plates coated with 5 μg/mL anti-CD3, treated with 20 μM rgal-1, briefly spun, incubated at 37°C for indicated time points, and fixed and stained as described. For gal-1−/− OT-1 thymocyte stimulations, 106 thymocytes were treated with 20 μM rgal-1, stimulated with 1 μg of SIINFEKL/Kb tetramers (Immunomics, Fullerton, CA) for 2 minutes at 37°C, and fixed and stained as described.
Tetramer staining decay assay
Assay and analysis were performed as described by Savage et al.26 Gal-1−/− OT-1 thymocytes were incubated with 0.1 μM rgal-1 for 5 minutes, washed gently, and stained with anti-CD4, -CD8, and SIINFEKL/Kb tetramers (Immunomics) for 45 minutes at room temperature. Anti-Kb antibody was added and aliquots were taken 0, 20, and 40 minutes afterward. Twenty-five thousand events per sample were immediately acquired. Total fluorescence was determined and normalized per DP thymocyte.
Results
Gal-1 opposed partial agonist-driven positive selection of conventional CD8+ T cells
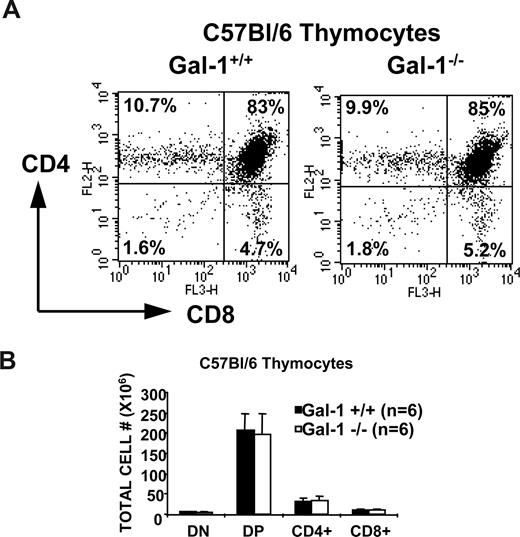
To determine whether gal-1 affects thymocyte development, we bred gal-1–deficient mice onto the C57Bl/6 background (referred to as gal-1−/− mice) and onto TCR transgenic strains. As expected, we did not detect gal-1 in thymi or spleens of gal-1−/− mice (Figure 1A,B). Gal-1 protein was detected throughout the thymus of wild-type mice (Figure 1A), essentially contacting every cell within the thymus. Gal-1−/− mice showed no abnormalities in thymic, splenic, or lymph node architecture, thymocyte and splenocyte cellular composition, or glycosylation profiles (Figures 1C,D, and 2, and data not shown). No differences in CD3, CD4, CD8, CD5, heat-stable antigen (HSA), CD69, CD62L, CD43, CD45, CD44, and B220 surface levels (not shown) or in PNA or SNA binding were observed between wild-type and gal-1−/− thymocytes (Figure 1C,D). Equivalent PNA and SNA binding suggested that potential gal-1 ligands were present on the thymocytes of gal-1−/− mice, ruling out potential secondary effects due to compensatory modulation of gal-1 ligands in the absence of its binding.
Gal-1 is expressed throughout the thymus, and ablation of the gal-1 gene did not affect glycosylation of thymocytes or thymic architecture. (A) Immunohistochemistry images of thymus from wild-type (top left) and gal-1−/− (top right) mice stained with a polyclonal anti–gal-1 antibody and counterstained with hemotoxylin. (B) Control immunohistochemistry images of thymus from wild-type (bottom left) and gal-1−/− (bottom right) mice stained with rabbit serum and counterstained with hematoxylin. (C,D) Flow cytometric histograms of SNA (C) and PNA (D) staining of wild-type (shaded) and gal-1−/− (thin line) DP (left), CD4+ SP (middle), and CD8+ SP (right) thymocyte populations. The numbers represent the mean fluorescent intensity of lectin staining in the wild-type (gray) and gal-1−/− (black) populations. Note the change in y-axis between histograms. Results represent 2 independent experiments.
Gal-1 is expressed throughout the thymus, and ablation of the gal-1 gene did not affect glycosylation of thymocytes or thymic architecture. (A) Immunohistochemistry images of thymus from wild-type (top left) and gal-1−/− (top right) mice stained with a polyclonal anti–gal-1 antibody and counterstained with hemotoxylin. (B) Control immunohistochemistry images of thymus from wild-type (bottom left) and gal-1−/− (bottom right) mice stained with rabbit serum and counterstained with hematoxylin. (C,D) Flow cytometric histograms of SNA (C) and PNA (D) staining of wild-type (shaded) and gal-1−/− (thin line) DP (left), CD4+ SP (middle), and CD8+ SP (right) thymocyte populations. The numbers represent the mean fluorescent intensity of lectin staining in the wild-type (gray) and gal-1−/− (black) populations. Note the change in y-axis between histograms. Results represent 2 independent experiments.
Thymocyte development was not perturbed in gal-1–deficient C57Bl/6 mice. (A) CD8 versus CD4 expression profiles of thymocytes from 6.2-week-old wild-type (left) and gal-1−/− (right) mice. The percentage of cells in each developmental subset is denoted in its respective quadrant. Flow profiles are representative of 6 independent mice. (B) The graph shows the mean of the absolute cell numbers (± SD) for each subpopulation, which was determined by multiplying the percentage of cells in each subset by the total number of thymocytes and averaging 6 mice per genotype.
Thymocyte development was not perturbed in gal-1–deficient C57Bl/6 mice. (A) CD8 versus CD4 expression profiles of thymocytes from 6.2-week-old wild-type (left) and gal-1−/− (right) mice. The percentage of cells in each developmental subset is denoted in its respective quadrant. Flow profiles are representative of 6 independent mice. (B) The graph shows the mean of the absolute cell numbers (± SD) for each subpopulation, which was determined by multiplying the percentage of cells in each subset by the total number of thymocytes and averaging 6 mice per genotype.
These data suggest that gal-1 is not essential for thymocyte development through the CD4+ or CD8+ SP stage. However, the effects of modulators of TCR-regulated thymocyte signaling and development are often masked in the context of a diverse TCR repertoire due to compensatory changes in the developing populations. Therefore, to better characterize gal-1 as a modulator of TCR-mediated thymic selection, we bred the gal-1 mutation onto 2 TCR transgenic mouse lines, H-Y and OT-1, which have been well characterized for the study of CD8+ T-cell development and positive and negative selection.27-37
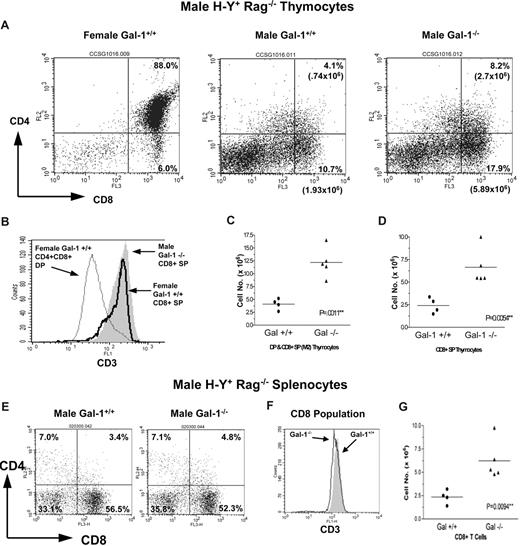
H-Y transgenic mice (Rag2−/−) exclusively express a TCR specific for a male H-Y antigen presented by the class I H-2Db protein. Thymocytes expressing H-Y specific TCRs are positively selected to develop along the CD8+ T-cell lineage in female H-2b mice, whereas H-Y TCR expressing thymocytes in male H-2b mice expressing the H-Y auto-antigen undergo deletion due to induced apoptosis during negative selection.28,29 We found that gal-1 ablation promoted positive selection of H-Y+CD8+ T cells in female mice. An increased number of CD8+ SP thymocytes was found in female gal-1−/− H-Y mice relative to wild-type littermate controls (Figure 3A,C). HSA and CD3 levels on DP and CD8+ SP thymocytes were comparable between female wild-type and gal-1−/− TCR transgenic T cells (Figure 3B), indicating that CD8+ SP thymocytes developing in gal-1−/− mice are mature. Spleens of female gal-1−/− H-Y mice exhibited an increase in the total number of H-Y+CD8+ T cells (Figure 3D,E), reflecting enhanced positive selection.
Gal-1 opposed partial agonist-driven positive selection of conventional CD8+ T cells. (A) CD8 versus CD4 expression profiles of thymocytes from wild-type (left) and gal-1−/− (right) female H-Y TCR transgenic mice. Percentage of cells in each subset is denoted in its respective quadrant, and the number of CD8+ SP thymocytes is in parentheses. (B) Flow cytometric histograms of CD3 (left) and HSA (right) staining of DP (top) and CD8+ SP (bottom) thymocytes from wild-type (shaded) and gal-1−/− (thin line) mice. Note change in y-axis between histograms. (C) The total number of CD8+ SP thymocytes from wild-type (n = 4) and gal-1−/− (n = 4) female H-Y TCR mice. Each point represents one mouse and was determined by multiplying the percentage of CD8+ SP thymocytes by the total number of thymocytes from each individual mouse throughout 4 independent experiments. The horizontal bar represents the mean. (D) Flow histograms of CD8 staining of splenocytes from wild-type (left) and gal-1−/− (right) female H-Y TCR mice. The numbers correspond to the percentage and total number of cells in the marker M1, which were set based on an isotype control and represent the CD8+ population. All dot plot profiles and histograms are representative of 4 independent experiments. (E) The total number of CD8+ T cells from spleens of wild-type (n = 4) and gal-1−/− (n = 4) female H-Y TCR mice. Each point represents one mouse, and the total number of CD8+ T cells was determined as described in panel C over the course of 4 independent experiments. (F) The total number of CD8+ SP thymocytes (left) and splenic CD8+ T cells (right) from wild-type (n = 4) and gal-1−/− (n = 5 for thymus, n = 4 for spleen) OT-1 TCR transgenic mice. Each point represents one mouse and was determined as described in panel C over 4 independent experiments. (G) Histograms of CD25 staining of unstimulated (left) and anti-CD3/anti-CD28–stimulated (right) enriched CD8+ T cells (80%-90% purity) from wild-type (shaded) and gal-1−/− (thin line) OT-1 TCR mice. Flow profiles are representative of 2 independent experiments. (H) Percent of CD8+ SP thymocytes from C57Bl/6 fetal thymii cultured (FTOC) in the presence ( and ■) or absence (□) of gal-1 inhibitor. (I) CD4:CD8 thymocyte ratio from a single FTOC experiment treated with or without gal-1 inhibitor. FTOC results are representative of 3 independent experiments. For panels C and E, statistical significance was determined by 2-tailed paired Student t tests. For panel F, one-tailed unpaired Student t tests were performed for OT-1 studies because these experiments were done to corroborate the female H-Y studies in which a hypothesis was previously established. P values are listed in panels.
and ■) or absence (□) of gal-1 inhibitor. (I) CD4:CD8 thymocyte ratio from a single FTOC experiment treated with or without gal-1 inhibitor. FTOC results are representative of 3 independent experiments. For panels C and E, statistical significance was determined by 2-tailed paired Student t tests. For panel F, one-tailed unpaired Student t tests were performed for OT-1 studies because these experiments were done to corroborate the female H-Y studies in which a hypothesis was previously established. P values are listed in panels.
Gal-1 opposed partial agonist-driven positive selection of conventional CD8+ T cells. (A) CD8 versus CD4 expression profiles of thymocytes from wild-type (left) and gal-1−/− (right) female H-Y TCR transgenic mice. Percentage of cells in each subset is denoted in its respective quadrant, and the number of CD8+ SP thymocytes is in parentheses. (B) Flow cytometric histograms of CD3 (left) and HSA (right) staining of DP (top) and CD8+ SP (bottom) thymocytes from wild-type (shaded) and gal-1−/− (thin line) mice. Note change in y-axis between histograms. (C) The total number of CD8+ SP thymocytes from wild-type (n = 4) and gal-1−/− (n = 4) female H-Y TCR mice. Each point represents one mouse and was determined by multiplying the percentage of CD8+ SP thymocytes by the total number of thymocytes from each individual mouse throughout 4 independent experiments. The horizontal bar represents the mean. (D) Flow histograms of CD8 staining of splenocytes from wild-type (left) and gal-1−/− (right) female H-Y TCR mice. The numbers correspond to the percentage and total number of cells in the marker M1, which were set based on an isotype control and represent the CD8+ population. All dot plot profiles and histograms are representative of 4 independent experiments. (E) The total number of CD8+ T cells from spleens of wild-type (n = 4) and gal-1−/− (n = 4) female H-Y TCR mice. Each point represents one mouse, and the total number of CD8+ T cells was determined as described in panel C over the course of 4 independent experiments. (F) The total number of CD8+ SP thymocytes (left) and splenic CD8+ T cells (right) from wild-type (n = 4) and gal-1−/− (n = 5 for thymus, n = 4 for spleen) OT-1 TCR transgenic mice. Each point represents one mouse and was determined as described in panel C over 4 independent experiments. (G) Histograms of CD25 staining of unstimulated (left) and anti-CD3/anti-CD28–stimulated (right) enriched CD8+ T cells (80%-90% purity) from wild-type (shaded) and gal-1−/− (thin line) OT-1 TCR mice. Flow profiles are representative of 2 independent experiments. (H) Percent of CD8+ SP thymocytes from C57Bl/6 fetal thymii cultured (FTOC) in the presence ( and ■) or absence (□) of gal-1 inhibitor. (I) CD4:CD8 thymocyte ratio from a single FTOC experiment treated with or without gal-1 inhibitor. FTOC results are representative of 3 independent experiments. For panels C and E, statistical significance was determined by 2-tailed paired Student t tests. For panel F, one-tailed unpaired Student t tests were performed for OT-1 studies because these experiments were done to corroborate the female H-Y studies in which a hypothesis was previously established. P values are listed in panels.
and ■) or absence (□) of gal-1 inhibitor. (I) CD4:CD8 thymocyte ratio from a single FTOC experiment treated with or without gal-1 inhibitor. FTOC results are representative of 3 independent experiments. For panels C and E, statistical significance was determined by 2-tailed paired Student t tests. For panel F, one-tailed unpaired Student t tests were performed for OT-1 studies because these experiments were done to corroborate the female H-Y studies in which a hypothesis was previously established. P values are listed in panels.
We extended our analysis of gal-1 effects on CD8+ thymocyte positive selection by examining gal-1 ablation in the context of another TCR transgene, the OT-1 TCR, specific for H-2b restricted OVA 257-264 and known to drive CD8+ T-cell development in C57Bl/6 mice.27 Again, we found that gal-1 ablation promoted positive selection of OT-1+CD8+ SP thymocytes (Figure 3F left) and development of mature OT-1+CD8+ T cells in the spleen (Figure 3F right). Further, mature CD8+ T cells from OT-1 gal-1−/− mice responded to anti-CD3/CD28 stimulation by up-regulating CD25 to the same extent as wild-type T cells, demonstrating functionality of positively selected T cells (Figure 3G). Our findings in both H-Y and OT-1 models support a role for gal-1 in opposing positive selection of CD8+ T cells.
This conclusion was further supported by experiments where addition of the gal-1–specific competitive inhibitor, dilactulose-hexamethylene diame (L2hmda),23,24 to fetal thymic organ culture (FTOC) of wild-type C57Bl/6 thymii led to selective enhancement of CD8+ T-cell development (Figure 3H,I). Under these circumstances, enhanced development of CD8+ T cells was apparent even in the absence of a TCR transgene. Presumably, restricting thymocyte development to a narrow window during FTOC does not allow compensatory processes that might mask gal-1 effects in diverse TCR backgrounds in vivo. Together, our findings support the conclusion that endogenous gal-1 opposes TCR partial agonist-driven positive selection of conventional CD8+ T cells.
Gal-1 promoted agonist-driven negative selection of CD8+ T cells in male H-Y TCR transgenic mice
We next extended our studies to determine the effects of gal-1 on negative selection of class I–restricted thymocytes. The male H-Y mouse exhibits a 5- to 10-fold decrease in the number of CD8+ SP thymocytes relative to female H-Y mice due to the deletion of H-Y+ thymocytes in response to male H-Y autoantigen during negative selection (Figures 3C,4C).28,29,33,38,39 Therefore, the male H-Y mouse represents an in vivo model for elucidating the role of endogenous gal-1 during CD8+ negative selection. We found that male gal-1−/− H-Y mice exhibited a 2.5- to3-fold increase in DP and CD8+ SP thymocytes (Figure 4A-D) and mature CD8+ splenocytes (Figure 4E-G), indicating that gal-1 expression promotes negative selection. Consistent with published literature, some H-Y thymocytes escape negative selection by down-regulating their CD8 surface levels, resulting in a population of CD8dull, but CD3hi, nonfunctional T cells (Figure 4A vs Figure 3A).28 To ensure that only CD8hi T cells were enumerated, flow cytometry gates were set based on isotype control and CD4/CD8 expression on thymocytes from a female H-Y mouse processed in parallel (Figure 4A,C,D). These data identify a role for endogenous gal-1 in promoting agonist-driven negative selection of CD8+ T cells. Together, our findings indicate that gal-1 ablation has opposing effects on positive versus negative selection of CD8+ T cells. While gal-1 promotes agonist-driven negative selection of CD8+ T cells, it antagonizes partial agonist-mediated positive selection of CD8+ T cells.
Gal-1 promoted agonist-driven negative selection of CD8+ T cells in male H-Y TCR transgenic mice. (A) CD8 versus CD4 expression profiles of thymocytes from wild-type (middle) and gal-1−/− (right) male H-Y TCR transgenic mice and a wild-type female H-Y mouse (left) processed in parallel. The percentage and total number of thymocytes are shown in the boxed region and represent the DP and CD8+ SP populations. (B) Flow cytometric histograms of CD3 staining of DP (thin line) and CD8+ SP (thick line) thymocytes from a control wild-type female H-Y mouse and CD8+ SP thymocytes from a gal-1−/− male H-Y mouse (shaded). (C, D) The total number of DP/CD8+ SP (C) and CD8+ SP (D) thymocytes from wild-type (n = 4) and gal-1−/− (n = 5) male H-Y TCR mice. These populations were determined by setting quadrants based on a wild-type female H-Y TCR control (Figure 4A). Each point represents one mouse, and total cell number was determined as described in Figure 3C over the course of 4 independent experiments. The horizontal bar represents the mean. (E) CD8 versus CD4 expression profiles of splenic cells from wild-type (left) and gal-1−/− (right) male H-Y TCR mice. The percentage of cells in each subset is shown in its respective quadrant. (F) Flow histogram of CD3 staining of CD8+ T cells from wild-type (shaded) and gal-1−/− (thin line) mice. (G) The total number of CD8+ T cells from spleens of wild-type (n = 4) and gal-1−/− (n = 5) male H-Y TCR mice. The total number of CD8+ T cells was determined as described in Figure 3, and each point represents one mouse over the course of 4 independent experiments. All flow profiles are representative of 4 independent experiments. For panels C, D, and G, 2-tailed unpaired Student t tests were performed to determine statistical significance. P values are listed in the panels.
Gal-1 promoted agonist-driven negative selection of CD8+ T cells in male H-Y TCR transgenic mice. (A) CD8 versus CD4 expression profiles of thymocytes from wild-type (middle) and gal-1−/− (right) male H-Y TCR transgenic mice and a wild-type female H-Y mouse (left) processed in parallel. The percentage and total number of thymocytes are shown in the boxed region and represent the DP and CD8+ SP populations. (B) Flow cytometric histograms of CD3 staining of DP (thin line) and CD8+ SP (thick line) thymocytes from a control wild-type female H-Y mouse and CD8+ SP thymocytes from a gal-1−/− male H-Y mouse (shaded). (C, D) The total number of DP/CD8+ SP (C) and CD8+ SP (D) thymocytes from wild-type (n = 4) and gal-1−/− (n = 5) male H-Y TCR mice. These populations were determined by setting quadrants based on a wild-type female H-Y TCR control (Figure 4A). Each point represents one mouse, and total cell number was determined as described in Figure 3C over the course of 4 independent experiments. The horizontal bar represents the mean. (E) CD8 versus CD4 expression profiles of splenic cells from wild-type (left) and gal-1−/− (right) male H-Y TCR mice. The percentage of cells in each subset is shown in its respective quadrant. (F) Flow histogram of CD3 staining of CD8+ T cells from wild-type (shaded) and gal-1−/− (thin line) mice. (G) The total number of CD8+ T cells from spleens of wild-type (n = 4) and gal-1−/− (n = 5) male H-Y TCR mice. The total number of CD8+ T cells was determined as described in Figure 3, and each point represents one mouse over the course of 4 independent experiments. All flow profiles are representative of 4 independent experiments. For panels C, D, and G, 2-tailed unpaired Student t tests were performed to determine statistical significance. P values are listed in the panels.
Gal-1 promoted agonist-driven selection of CD8αα IEL
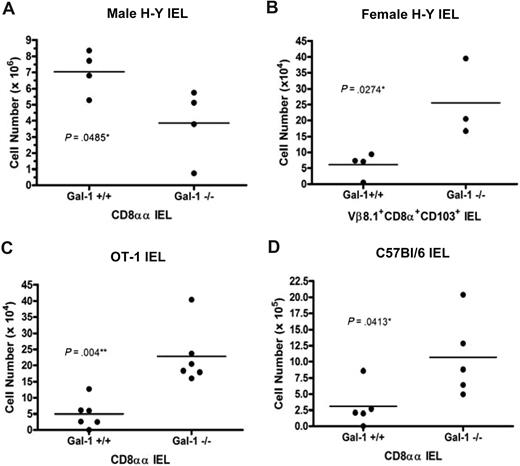
Recent studies have led to the identification of regulatory T-cell populations bearing autoreactive TCRs. These include α/β TCR CD8αα intestinal IEL, which preferentially migrate to the gut after positive selection on agonist ligands in the thymus.40 H-Y and OT-1 mice have been well characterized with regard to CD8αα IEL development.3,41 CD8αα IEL are more efficiently selected in agonist antigen expressing male H-Y mice than in partial agonist expressing female H-Y mice.41 Similarly, because OT-1 mice do not express agonist antigen, only a few CD8αα IEL develop.3 Because gal-1 promoted agonist-driven negative selection of conventional H-Y T cells during thymocyte development, we hypothesized that gal-1 expression might similarly promote agonist-driven CD8αα IEL development. Thus, we extended our studies to assess the effects of gal-1 expression on agonist-driven selection of H-Y+ CD8αα IEL in male H-Y mice. To this end, IEL were isolated from the small intestine of wild-type and gal-1−/− male H-Y mice. CD8αα IEL were distinguished from other intestinal cell populations by gating on cells expressing CD8α, but not CD8β. All CD8α+CD8β− cells expressed the CD103 surface marker and were transgene Vβ8.2+ (not shown). We found a decrease in the number of H-Y+ CD8αα IEL in male gal-1−/− H-Y mice relative to wild-type controls (Figure 5A). Therefore, gal-1 promotes the well-characterized agonist-driven selection of CD8αα IEL in male H-Y mice.
Gal-1 promoted agonist-driven positive selection of CD8αα IEL. (A,C,D) The total number of CD8αα (CD8α+CD8ββCD103+) IELs from wild-type (n = 4) and gal-1−/− (n = 4) male H-Y TCR transgenic mice (A); wild-type (n = 6) and gal-1−/− (n = 6) OT-1 TCR transgenic mice (C); and wild-type (n = 5) and gal-1−/− (n = 5) C57Bl/6 mice (D). (B) The total number of TCR transgenic positive CD8α+CD103+ IELs from wild-type (n = 4) and gal-1−/− (n = 3) female H-Y TCR transgenic mice. More than 75% of CD8α+CD103+ IEL were CD8β−. Each point represents one mouse and was determined by multiplying the percentage of H-Y positive CD8αα, OT-1 positive CD8αα, or C57Bl/6 CD8αα lymphocytes by the total number of isolated IELs for each individual mouse. The horizontal bar represents the mean. For panel A, statistical significance was determined by 2-tailed paired Student t tests. For panels B through D, statistical significance was determined by 2-tailed unpaired Student t tests. P values are listed in the panels.
Gal-1 promoted agonist-driven positive selection of CD8αα IEL. (A,C,D) The total number of CD8αα (CD8α+CD8ββCD103+) IELs from wild-type (n = 4) and gal-1−/− (n = 4) male H-Y TCR transgenic mice (A); wild-type (n = 6) and gal-1−/− (n = 6) OT-1 TCR transgenic mice (C); and wild-type (n = 5) and gal-1−/− (n = 5) C57Bl/6 mice (D). (B) The total number of TCR transgenic positive CD8α+CD103+ IELs from wild-type (n = 4) and gal-1−/− (n = 3) female H-Y TCR transgenic mice. More than 75% of CD8α+CD103+ IEL were CD8β−. Each point represents one mouse and was determined by multiplying the percentage of H-Y positive CD8αα, OT-1 positive CD8αα, or C57Bl/6 CD8αα lymphocytes by the total number of isolated IELs for each individual mouse. The horizontal bar represents the mean. For panel A, statistical significance was determined by 2-tailed paired Student t tests. For panels B through D, statistical significance was determined by 2-tailed unpaired Student t tests. P values are listed in the panels.
To determine whether endogenous gal-1 modulates CD8αα IEL development in partial agonist-expressing backgrounds, IEL from gal-1−/− female H-Y and OT-1 mice were analyzed. Consistent with published results, few CD8αα IELs were isolated from wild-type female H-Y and OT-1 mice (Figure 5B,C).3 However, significantly more Vβ8.2+CD8α+CD103+ IEL were found in female H-Y gal-1−/− mice (Figure 5B). Similarly, more intestinal Vα2+CD8α+CD8β−CD103+ IEL were identified in OT-1 mice relative to wild-type controls (Figure 5C). We also found an increased number of CD8αα IEL (Figure 5D) in gal-1−/− C57Bl/6 mice relative to wild-type mice. Like conventional CD8 T-cell development, gal-1 oppositely modulates the number of mature IEL present in the context of agonist versus partial agonist ligands. This may reflect a role for gal-1 in promoting partial agonist-driven selection of IEL or, alternatively, IEL intestinal homing.
Gal-1 promoted TCR-agonist/MHC binding and intensified the transient negative selection ERK signaling signature
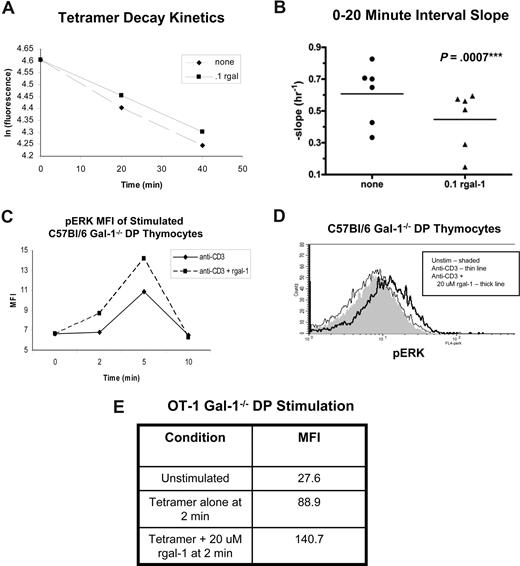
Previous studies have used tetramer staining decay assays to determine that peptide/MHC tetramer binding to peptide-specific cells correlates with the affinity of the TCR for the peptide/MHC complex.26,42-44 Because gal-1 has been demonstrated to bind several thymocyte surface ligands, including TCR-associated CD3, we explored the possibility that gal-1 might modulate TCR agonist ligand binding or signaling. Therefore, we performed tetramer decay assays using agonist SIINFEKL/Kb tetramers and gal-1−/− OT-1 thymocytes in the absence or presence of rgal-1. Gating on tetramerhi DP thymocytes, we first obtained linear decay plots of the natural logarithm of fluorescence versus time (Figure 6A). Further quantitative analysis showed that plots from rgal-1 treated DP thymocytes had a smaller negative slope relative to untreated thymocytes at the 0-20 time minute interval, indicating that gal-1 increases the binding of the OT-1+ DP thymocytes to agonist tetramers (Figure 6B). This increase (∼40%) in binding is within the general range of physiologically relevant differences measured among developing thymocyte, primary T-cell, and secondary T-cell TCR repertoires, used to document narrowing of specificities as a result of TCR selection events.26,42 Conversely, rgal-1 did not significantly affect binding of agonist tetramers to the TCR on CD8+ SP thymocytes (not shown). This is consistent with terminal sialylation blocking gal-1 binding to thymocytes as they transition from DP to CD8+ SP thymocytes.8,43 These data suggest that gal-1 enhances binding of the TCR on developing DP thymocytes to agonist peptide/MHC complexes, thus enforcing and promoting agonist signaling and fate decisions.
Gal-1 promoted TCR-agonist/MHC binding and intensified the transient negative selection ERK signaling signature. Tetramer staining decay kinetics for gal-1−/− OT-1 DP thymocytes incubated with or without 0.1 μM rgal-1 and SIINFEKL/Kb tetramer (A,B). (A) Plot of the natural logarithm of normalized fluorescence versus time after anti-Kb addition of untreated (■) and rgal-1–treated (♦) gal-1−/− OT-1 DP thymocytes. (B) Quantitative slope analysis of tetramer staining decay kinetics at the 0-20 minute interval between rgal-1–treated (▴, n = 6) and untreated (•, n = 6) DP populations. The slope was determined by ln (Fa/Fb)/t, where Fa is total fluorescence at the beginning of the interval and Fb is fluorescence at the end of the interval, and t is time, in hours, of interval. The horizontal bar represents the mean. To determine statistical significance, paired 2-tailed Student t tests were performed between untreated and rgal-1–treated samples. P value is listed in the panel. (C) The intensity of pERK MFI in agonist anti-CD3 antibody stimulated gal-1−/− DP thymocytes from C57Bl/6 mice treated with (broken line) or without (solid line) 20 μM rgal-1. (D) Histograms of pERK staining in DP thymocytes from gal-1−/− C57Bl/6 mice stimulated with anti-CD3 antibody for 2 minutes in the absence (thin line) or presence of 20 μM rgal-1 (thick line) or unstimulated (shaded). Results represent 4 independent experiments. (E) The mean fluorescent intensity (MFI) of pERK in untreated or 20 μM rgal-1–treated gal-1−/− OT-1 DP thymocytes stimulated with agonist SIINFEKL/Kb tetramers for 2 minutes.
Gal-1 promoted TCR-agonist/MHC binding and intensified the transient negative selection ERK signaling signature. Tetramer staining decay kinetics for gal-1−/− OT-1 DP thymocytes incubated with or without 0.1 μM rgal-1 and SIINFEKL/Kb tetramer (A,B). (A) Plot of the natural logarithm of normalized fluorescence versus time after anti-Kb addition of untreated (■) and rgal-1–treated (♦) gal-1−/− OT-1 DP thymocytes. (B) Quantitative slope analysis of tetramer staining decay kinetics at the 0-20 minute interval between rgal-1–treated (▴, n = 6) and untreated (•, n = 6) DP populations. The slope was determined by ln (Fa/Fb)/t, where Fa is total fluorescence at the beginning of the interval and Fb is fluorescence at the end of the interval, and t is time, in hours, of interval. The horizontal bar represents the mean. To determine statistical significance, paired 2-tailed Student t tests were performed between untreated and rgal-1–treated samples. P value is listed in the panel. (C) The intensity of pERK MFI in agonist anti-CD3 antibody stimulated gal-1−/− DP thymocytes from C57Bl/6 mice treated with (broken line) or without (solid line) 20 μM rgal-1. (D) Histograms of pERK staining in DP thymocytes from gal-1−/− C57Bl/6 mice stimulated with anti-CD3 antibody for 2 minutes in the absence (thin line) or presence of 20 μM rgal-1 (thick line) or unstimulated (shaded). Results represent 4 independent experiments. (E) The mean fluorescent intensity (MFI) of pERK in untreated or 20 μM rgal-1–treated gal-1−/− OT-1 DP thymocytes stimulated with agonist SIINFEKL/Kb tetramers for 2 minutes.
Accumulating data indicate differential activation and kinetics of MAP kinase cascades as central to determining TCR-driven thymocyte functional fate decisions.5,6 Positive selection relies on low-grade sustained ERK activation induced by partial agonist TCR ligands, whereas agonist ligands capable of negative selection induce an intense burst of ERK activation, peaking within 1 to 5 minutes of receptor engagement.6 These differences reflect TCR coupling to alternate routes of ERK activation in distinct subcellular locations.5 To determine whether gal-1 was selectively modulating TCR signal transduction pathways associated with thymocyte developmental cues, we assessed the effects of gal-1 on TCR-induced ERK activation in positive and negative selection models.
To assess gal-1 contributions to transient TCR-driven ERK activation during negative selection, we turned to an established in vitro model of negative selection wherein anti-CD3 engagement induces apoptosis in freshly isolated thymocyte cultures.44 Thymocytes from gal-1−/− or wild-type mice were stimulated with anti-CD3 antibody in the presence or absence of rgal-1, and levels of activated/phosphorylated ERK (pERK) were assessed using flow cytometry. Anti-CD3 stimulation mimicked negative-selecting ligands, inducing transient ERK activation in DP thymocytes and peaking within the first 2 to 5 minutes (Figure 6C,D). Consistent with our findings that gal-1 expression facilitated negative selection in male H-Y TCR transgenic mice, addition of rgal-1 enhanced the intensity of anti–CD3-induced transient ERK signaling, but did not alter kinetics, in both wild-type and gal-1−/− DP thymocytes (Figure 6, and not shown). These findings are in keeping with our previously published biochemical analysis demonstrating that rgal-1 enhances ERK kinase activity in thymocytes treated with agonist anti-CD3 antibody, validating our flow cytometry–based assay.19 Similarly, agonist tetramer-induced ERK activation of OT-1 thymocytes was intensified within 2 minutes of TCR/gal-1 engagement (Figure 6E). For these studies analyzing TCR signaling generated in vitro, we attempted to minimize the contributions of endogenous gal-1 and TCR signals generated in vivo by extensive washing and resting of the thymocytes in vitro, and synchronously exposing these thymocytes to rgal-1 and TCR ligand. These findings support the hypothesis that gal-1 enhances TCR binding to agonist/MHC complexes, intensifying a negative selection ERK signaling signature in developing thymocytes and promoting negative selection.
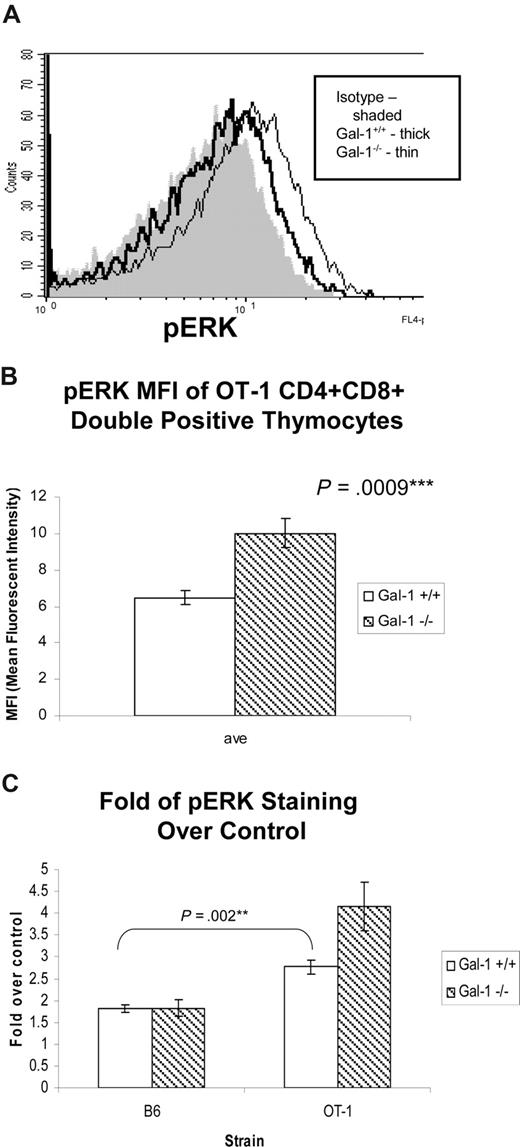
In contrast to the transient ERK activation in thymocytes undergoing negative selection, the low-intensity ERK activation associated with positive selection is sustained for at least 1 hour after receptor engagement.6 Therefore, we reasoned that we might detect ERK signaling in thymocytes isolated directly ex vivo from a positive selecting environment. To this end, we isolated and immediately fixed thymocytes from wild-type and gal-1−/− OT-1 mice and assessed them for the presence of pERK. pERK was detected in both wild-type and gal-1−/− OT-1 thymocytes when compared with isotype control (Figure 7). Consistent with our findings that gal-1 expression antagonizes positive selection, more pERK was detected in gal-1−/− DP OT-1 thymocytes than in wild-type thymocytes (Figure 7A,B). Because the OT-1 TCR drives increased number of thymocytes to undergo positive selection relative to C57Bl/6 mice, we hypothesized that more pERK would be present in OT-1 DP thymocytes relative to C57Bl/6 DP thymocytes. Indeed, we found more pERK in OT-1 thymocytes (Figure 7C), further establishing a correlation between ERK activation in freshly fixed thymocytes and the degree of positive selection. That differences between wild-type and gal-1−/− were only appreciated in the context of the positive selecting OT-1 TCR transgene further validate this model. These findings indicate that gal-1 can antagonize ERK activity in thymocytes undergoing positive selection.
Gal-1 antagonized ERK signaling in thymocytes undergoing positive selection. (A) Histogram of phospho-ERK (pERK) staining in DP thymocytes from wild-type (thick line) and gal-1−/− (thin line) OT-1 mice, and isotype control (shaded). Cells were immediately fixed and permeabilized after dissociation from thymic stroma to preserve positive selecting signals induced in vivo. (B) The average MFI (± SD) of pERK in DP thymocytes from 3 wild-type (□) and 4 gal-1−/− (▧) OT-1 TCR mice. Results represent 2 independent experiments. (C) The average of fold over control (± SD) of pERK staining in wild-type (□) and gal-1−/− (▧) DP thymocytes from C57Bl/6 (wild-type, n = 4; gal-1−/−, n = 4) and OT-1 TCR transgenic mice (wild-type, n = 4; gal-1−/−, n = 5). For panels B and C, 2-tailed unpaired Student t tests were performed to determine statistical significance. P values are listed in the panels.
Gal-1 antagonized ERK signaling in thymocytes undergoing positive selection. (A) Histogram of phospho-ERK (pERK) staining in DP thymocytes from wild-type (thick line) and gal-1−/− (thin line) OT-1 mice, and isotype control (shaded). Cells were immediately fixed and permeabilized after dissociation from thymic stroma to preserve positive selecting signals induced in vivo. (B) The average MFI (± SD) of pERK in DP thymocytes from 3 wild-type (□) and 4 gal-1−/− (▧) OT-1 TCR mice. Results represent 2 independent experiments. (C) The average of fold over control (± SD) of pERK staining in wild-type (□) and gal-1−/− (▧) DP thymocytes from C57Bl/6 (wild-type, n = 4; gal-1−/−, n = 4) and OT-1 TCR transgenic mice (wild-type, n = 4; gal-1−/−, n = 5). For panels B and C, 2-tailed unpaired Student t tests were performed to determine statistical significance. P values are listed in the panels.
Discussion
Using well-characterized class I–restricted TCR transgenic mouse models, we found that endogenous gal-1 opposes positive selection and promotes negative selection of conventional CD8+ T cells. In positive selection models, gal-1–deficient mice were more efficient at positive selection than wild-type littermates, as reflected by the development of more CD8+ SP TCR transgenic thymocytes and splenocytes. We did not observe aberrant cellularity or percentages of DP, CD4+, or CD8+ conventional T-cell subsets in gal-1–deficient mice in the absence of a TCR transgene. Therefore, gal-1 is not essential for T-cell development, nor does it regulate thymic cellularity, in general. Rather, the effects of gal-1 ablation only became apparent in the context of thymocytes bearing a fixed and tractable TCR, supporting the suggestion that gal-1 modulates TCR-driven thymocyte development.
Experiments capitalizing on FTOC and the gal-1–specific inhibitor, L2hmda, provide additional support for gal-1 in opposing CD8+ positive selection using an independent approach. The apparently selective effects of gal-1 on CD8+, but not CD4+, development in FTOC raise the possibility that gal-1 may play a greater role in modulating positive selection of CD8+ versus CD4+ T cells. While we did not observe selective effects on CD8 versus CD4 development in gal-1–deficient mice in the absence of a TCR transgene, restricting thymocyte development to a narrow window in FTOC might prevent compensatory mechanisms, such as thymocyte repopulation and egress, which mask the effects of gal-1 in diverse TCR backgrounds in vivo. Alternatively, L2hmda may be affecting another galectin family member expressed in the thymus with overlapping specificity, such as galectin-9,45 which may independently or additionally contribute to T-cell lineage commitment and/or selection. Ongoing studies analyzing gal-1 ablation in the context of class II–restricted TCR transgenes on positive selecting backgrounds should lend insight into this issue.
By analyzing gal-1 ablation in the context of the male H-Y model of negative selection, we found that gal-1 promotes negative selection of conventional CD8+ T cells. This was reflected by the development of more DP and CD8+ SP H-Y TCR thymocytes and splenocytes in gal-1–deficient versus wild-type males. Together, our findings indicate that gal-1 opposes class I–restricted thymocyte positive selection, but promotes negative selection. In the transgenic models studied, gal-1 ablation leads to a 2- to 3-fold difference in the number of thymocytes developing on both positive and negative selecting backgrounds. This level of perturbation of thymocyte development is similar to that observed in the context of ablating known physiologically relevant modulators in the same transgenic models.32,33,46 However, because gal-1 has opposite effects on positive and negative selection, the combined effects of gal-1 on thymocyte development is predicted to be even greater.
Our findings led us to consider the possibility that gal-1 might distinguish IEL development in the context of agonist-directed selection. We found that gal-1 promoted agonist IEL selection in H-Y male mice. Therefore, in H-Y thymocytes, gal-1 promoted agonist-driven conventional CD8 T-cell and CD8αα IEL selection. Alternately, we found fewer IEL in gal-1–deficient female H-Y and OT-1 TCR mice, known to express partial agonist ligands. This may reflect a role for gal-1 in antagonizing suboptimal selection by partial agonists for CD8αα IEL. However, a role for partial agonist binding in selection of IEL has yet to be described. Alternatively, gal-1 could be controlling homing of CD8αα IEL to the intestine, consistent with studies implicating gal-1 in T-cell trafficking during inflammation.47
We previously reported that gal-1 has the capacity to differentially regulate distinct downstream TCR signal transduction events and functional outcomes.18 Gal-1 may alter TCR coupling to downstream signaling pathways (including ERK activation) by positioning surface glycoprotein or glycolipid ligands capable of influencing TCR binding and signal transduction. Alternatively, intracellular gal-1 might function to regulate ERK activation pathways by influencing Ras subcellular localization, as has been described in other cell types.48 The addition of extracellular rgal-1 is predicted to reverse, whereas L2hmda is predicted to mimic, the effects of gal-1 deficiency in binding surface glycoproteins. These perturbations should not affect intracellular gal-1 activity. Because data using rgal-1, L2hmda, and gal-1–deficient mice all support opposing contributions of gal-1 to positive and negative selection, we currently favor a model whereby gal-1 contributes to TCR signaling through its ability to bind cell surface glycoproteins or glycolipids and impact TCR ligand binding, packing, and signaling.
Emerging models of TCR signal specificity posit that the organized immune synapse functions to provide context for the TCR, fine tuning TCR engagement, signal transduction, and coupling signals to downstream function.49 Alternate synaptic organization has been reported in T-cell developmental subsets and is believed to contribute to modulation of TCR signal specificity.49 Gal-1 T-cell counter-ligands include CD45, CD43, GM1, CD3, CD4, and CD7, each known to modulate TCR signal transduction and to be dynamically repositioned during the course of T-cell stimulation, synaptogenesis, and signal transduction in response to antigen.14,17,18,50-52 Further, gal-1 can selectively induce the formation of segregated surface glycolattice-based microdomains including CD45/CD3 or CD43/CD7 in CD3lo thymocytes.14,20,53 CD3, CD43, and/or CD45 represent interesting candidate glycoprotein counter-ligands required for gal-1–negative selecting activity since they have each been implicated in promoting thymocyte-negative selection, apoptosis, and ERK activation.54-60
Gal-1 can also bind the raft glycolipid GM1 and reorganize lipid raft microdomain distribution at the TCR contact site.18 Translocation of the TCR α-chain and CD3δ to lipid rafts is required for positive, but not negative, selection and raft microdomain partitioning can regulate CD45-mediated ERK activation.6,59,60 We found that gal-1 promotes TCR binding to agonist/MHC ligand, a phenomenon which could reflect increased stabilization of agonist-induced TCR confirmations or packing of thymocyte surface glycolattices/membrane microdomains that favor TCR agonist binding and/or impair its disassociation. Based on our collective findings, we hypothesize that gal-1 functions as a scaffold that orchestrates preferential TCR agonist binding and coupling to downstream transient ERK activation and signaling by reorganizing glycoprotein and/or raft-based microdomains at the TCR contact site to enforce TCR fate decisions. Elucidation of the molecular basis of gal-1 activity on partial agonist TCR binding and signaling will aid in refining this hypothesis.
While it has yet to be firmly established which TCR-mediated signals combine to specify negative versus positive selection, accumulating data implicate differential activation and kinetics of MAP kinase cascades as central to determining these functional fate decisions.53 Whereas both positive and negative selecting ligands induce ERK, JNK, and p38 activity, positive and negative selection differentially rely on their activities.53,61-64 Negative selecting (agonist) ligands induce a strong and immediate peak in ERK activation by coupling linker for T-cell activation (LAT) phosphorylation to both Grb2:SOS and Ras guanyl nucleotide-releasing protein (RasGRP)–mediated ERK activation at the plasma membrane.5,6 Alternatively, positive-selecting (partial agonist) ligands induce less intense, more sustained ERK activation by coupling to phospholipase C (PLC)γ–mediated diacylglycerol (DAG) production and activation of RasGRP in the Golgi.5,6 There is consensus that positive selection requires RasGRP-mediated ERK activity,53,65,66 whereas the contribution of Grb2:Sos-mediated ERK activity to negative selection is more controversial.53,61,62,64 Some evidence suggests that JNK and p38 activation may be especially important for negative selection.46,63,64
We have reported that rgal-1 cooperates with agonist anti-TCR engagement to enhance apoptosis and ERK activation in thymocytes within 1 minute of activation and peaking within 5 minutes.19 These kinetics closely mirror the recently identified ERK signature of negative-selecting ligands6 and are consistent with our findings that gal-1 promotes negative selection in vivo. Here, we further explored the ability of gal-1 to modulate pERK intensity in the context of defined positive and negative selection models. Consistent with gal-1 promotion of negative selection, we found that gal-1 intensifies transient ERK signaling in DP thymocytes in the anti-CD3 in vitro negative selection model and in response to agonist peptide/MHC. Conversely, gal-1 expression antagonized ERK activation in freshly isolated thymocytes undergoing positive selection, where ERK activity was positively correlated with the degree of positive selection in gal-1–deficient and TCR transgenic populations. Perhaps gal-1 binding in the context of agonist and partial agonist TCR ligands differentially modulates Grb:Sos versus RasGRP signaling pathways, thus impacting functional fate decisions. Future studies capitalizing on the behavior of well-defined OT-1 TCR agonist and partial agonist ligands (on β2M null and gal-1–deficient backgrounds) should enable us to test these ideas and characterize the molecular basis of gal-1 activity on partial agonist signaling to further elucidate the mechanism by which gal-1 guides functional fate decisions in the thymus.
Here, we identify gal-1 as a unique thymocyte TCR modulator with opposing activities on thymocytes recognizing qualitatively distinct TCR ligands. We provide evidence that gal-1 selectively promotes negative selection and opposes positive selection and enforces distinctions in MAP kinase activation. Thus, our findings to date indicate that gal-1 increases the threshold for positive selection, while lowering the threshold for negative selection. For peptides at the border of positive selection versus negative selection, gal-1 activity is predicted to sharpen and amplify subtle distinctions, enabling absolute functional fate decisions with a bias toward ensuring self-tolerance. For peptides at the border of positive versus nonselection, gal-1 activity is predicted to narrow the repertoire, favoring the development of T cells with maximal use. These findings highlight a novel role for glycosylation and endogenous lectin binding in regulating TCR signal specificity and enforcing thymocyte fate determination.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Hilde Cheroutre for providing us with the H-Y and OT-1 TCR mice, and Daniel Cruz, Peter Krutzik, and the laboratory of Ken Dorshkind for technical assistance. We also thank members of the Miceli Lab for critical reading of the manuscript.
This work was supported by National Institutes of Health (NIH) grant RO1A1056155 to M.C.M. S.D.L. was supported by the Microbial Pathogenesis Training Grant T32 AI07323-15, Clinical and Fundamental Training Grant AI07126-30, and Warsaw Fellowship. T.T. and S.J.B. are recipients of the Microbial Pathogenesis Training Grant 2-T32-AI-07323. F.P. received financial support from Association pour la Recherche sur le Cancer and Ligue Contre le Cancer. L.G.B. was funded by NIH R01 GM63281. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility, supported by NIH Awards CA-16042 and AI-28697.
National Institutes of Health
Authorship
Contribution: S.D.L. designed and performed research, analyzed data, and wrote the paper. C.C.W. designed and performed research, and analyzed data. T.T. performed research and analyzed data. M.P. performed research. S.J.B. assisted with research. V.V.M. provided vital reagents. F.P. provided the galectin-1–deficient mice. L.G.B. provided reagents and insight. M.C.M. designed research, provided insight, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Carrie Miceli, Department of Microbiology, Immunology, and Molecular Genetics, 277B Biological Science Research Bldg, University of California, Los Angeles, School of Medicine, 615 Charles E Young Dr East, Los Angeles, CA 90095-1570; e-mail: cmiceli@ucla.edu.