Abstract
Bruton tyrosine kinase (Btk) is critical for B-cell development. Btk regulates a plethora of signaling proteins, among them nuclear factor-[κ]B (NF-κB). Activation of NF-κB is a hallmark of B cells, and NF-κB signaling is severely compromised in Btk deficiency. We here present strong evidence indicating that NF-κB is required for efficient transcription of the Btk gene. First, we found that proteasome blockers and inhibitors of NF-κB signaling suppress Btk transcription and intracellular expression. Similar to Btk, proteasome inhibitors also reduced the expression of other members of this family of kinases, Itk, Bmx, and Tec. Second, 2 functional NF-κB–binding sites were found in the Btk promoter. Moreover, in live mice, by hydrodynamic transfection, we show that bortezomib (a blocker of proteasomes and NF-κB signaling), as well as NF-κB binding sequence-oligonucleotide decoys block Btk transcription. We also demonstrate that Btk induces NF-κB activity in mice. Collectively, we show that Btk uses a positive autoregulatory feedback mechanism to stimulate transcription from its own promoter via NF-κB.
Introduction
Bruton tyrosine kinase (Btk) is a nonreceptor tyrosine kinase belonging to the Tec family of protein tyrosine kinases (PTKs). This family consists of 5 mammalian members: Btk, Itk, Tec, Bmx, and Txk. These kinases are also present in other species.1,2 Btk is expressed in almost all the hematopoietic cells, except T lymphocytes and plasma cells, and in the B-cell lineage, from the very early up to the mature cell stages.3-5 Btk is required for B-lymphocyte survival, proliferation, and differentiation in response to BCR activation. Accordingly, mutations in the gene coding for Btk lead to X-linked agammaglobulinemia (XLA) in humans6-9 and X-linked immunodeficiency (xid) in mice.10,11
Btk and its direct substrate, phospholipase Cγ2 (PLC-γ2), are essential for the activation of the transcription factor nuclear factor-κB (NF-κB) in response to BCR engagement.12-14 Moreover, Shinners et al recently found that Btk mediates B-cell–activating factor receptor (BAFF-R)–dependent activation of NF-κB, thereby promoting B-cell survival.15
NF-κB is known to be essential for both innate and adaptive immunity. Moreover, it is crucial for the initial responses of sentinel cells to pathogens, as well as for the subsequent events that lead to T cell– and B cell–mediated antigen-specific defense. The role of NF-κB in the acute, innate immune response is evolutionarily conserved.16 NF-κB has also been shown to be crucial for the development of several mammalian hematopoietic cell lineages and for the formation of secondary lymphoid-organ structures.
The NF-κB/Rel family of proteins include NF-κB1 (p50), NF-κB2 (p52), RelA (p65), c-Rel, and RelB, which can form functional homodimer or heterodimer complexes.16,17 In resting cells, NF-κB is sequestered in the cytoplasm by the inhibitory proteins of the I-κB family. Following stimulation of cells by inflammatory cytokines, and bacterial (eg, LPS) and viral products, the inhibitor of κB (I-κB) is phosphorylated by the I-κB kinases (IKKs), leading to its degradation through the ubiquitin-proteasome pathway. Thus, in the absence of I-κB, NF-κB dimers (p50-p65 and p52-RelB) are released, translocated to the nucleus, and subsequently bound to their cognate elements on target genes.18
Mice deficient in individual NF-κB/Rel family members have demonstrated the essential role of these transcription factors in CD40, TLR4, and IgM receptor pathways leading to B-cell proliferation. In particular, B cells from mice deficient in the NF-κB members c-Rel or p65 have decreased responses to antigen cross-linking.19,20 c-Rel−/− B cells also failed to respond to CD40 ligation. An examination of B cells in mice expressing a transdominant form of I-κBα revealed xid-like defects, including lack of proliferation in response to anti-IgM.21
The transcriptional regulation of the Btk gene has been studied quite extensively. Accordingly, a number of transcription factors including Sp1, Sp3, Spi-B, PU.1,22-24 and OCT1/OBF125 have been shown to bind and activate the Btk promoter. However, most previously reported studies focused on the core promoter region (within −300 region) of the Btk gene. In PU.1-deficient fetal liver cells as well as in Sp1−/− embryonic stem cells, although expression of Btk was reduced, it was not completely abolished.23,24 This indicates that additional factors also contribute to Btk promoter activity. In this study, we show that NF-κB signaling directly regulates Btk expression. Furthermore, we herein demonstrate that Btk induces its own promoter via the NF-κB signaling pathway.
Methods
Reagents
Anti-Btk antibodies, and protease and phosphatase inhibitors have been described.26 MG132, lactacystin, ALLN, epoxomicin, cycloheximide, and Bay 11-7085 were purchased from Sigma-Aldrich (St Louis, MO). Bortezomib was obtained from Millennium Pharmaceuticals (Cambridge, MA). Polyclonal anti-p50, anti-p65, anti-PU.1, anti-p53, anti-Lyn, anti-Syk, anti-Tec, anti-Bmx, anti-Itk, anti-ERK1 and 2, and rabbit normal IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). NE-PER nuclear and cytoplasmic extraction kit was from Pierce (Rockford, IL).
Cell culture and transfections
Nalm6, Ramos, K562, and Jurkat cells were maintained in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS) and 1% PEST (Invitrogen, Carlsbad, CA). Human umbilical vein endothelial cells (Huvecs) were isolated as described.27 Cells were cultured in Huvec CM (M199 containing l-glutamine and 10% FCS, 30 μg/mL endothelial cell growth supplement [Sigma-Aldrich], 90 μg/mL heparin, and antibiotics). The chicken B-cell lines DT40, B7-10 (Btk-deficient DT40), and B41-13 (B7-10 reconstituted with human Btk) were kindly provided by Dr Tomohiro Kurosaki.28 Cells were maintained in RPMI 1640 with 10% FCS, 1% chicken serum, 2 mM glutamine, 1% PEST, 50 μM 2-mercaptoethanol, and antibiotics. HEK 293T, COS-7, and A20 cells were cultured and transfected as previously described.29
Plasmid constructs and luciferase assay
Constructs encoding Btk-GFP and E41K-Btk were described previously.30 The Btk promoter constructs 1000-Btk (−1049/+91) and 500-Btk (−511/+91) were amplified by polymerase chain reaction (PCR) using genomic DNA, and verified by sequencing. The PCR fragment was digested with BglII and HindIII and subcloned into the pGL3-Basic vector. PCR primers for 1000-Btk were forward, 5′-TCACTAGATCTGCACCTTCTGCACATATACC-3′ and reverse, 5′-AGTGAAAGCTTTGAGATGCCAGGACTTGGAA-3′. Primers for 500-Btk were forward, 5′-AGTTCAGATCTGGAAGTCTTGTCTCTACCTC-3′ and reverse, 5′-AGTGAAAGCTTTGAGATGCCAGGACTTGGAA-3′. Site-directed mutagenesis was used to generate mutant versions of the 1000-Btk reporter construct. The 1000-Btk-Mu 1 (NF-κB–binding site 1 mutant) was created by changing the NF-κB–binding sequence AATGGGGAAGGG to AATGTTTAAGGG, whereas the 1000-Btk-Mu 2 (NF-κB–binding site 2 mutant) was made by changing the NF-κB–binding sequence AGGAGAATCCCTTT to ATTTTAATCCCTGG. Finally, the 1000-Btk-Mu-(1 + 2) was made by combining mutants 1 and 2. All constructs were verified by DNA sequencing. Plasmid pcDNA1-p65 (full-length human p65/RelA expressed from a cytomegalovirus [CMV] promoter) was from Rune Toftgård (Karolinska Institutet, Stockholm, Sweden). The NF-κB reporter construct, pNF-κB-Luc, was purchased from Clontech (Palo Alto, CA). Luciferase activity was measured by the BioThema kit (Dalaro, Sweden) according to the manufacturer's instruction, and luminescence was measured in a microplate luminometer FLUOstar OPTIMA (BMG Labtech, Offenburg, Germany).
RT-PCR and quantitative RT-PCR
Total RNA was isolated using Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany). The reverse transcription (RT) and PCR were performed using Qiagen OneStep RT-PCR kit. Primers for Btk were forward, 5′-GAAGCTGGTGCAGTTGTATG-3′ and reverse, 5′-TATACCCTCTCTGATGCCAG-3′; primers for GAPDH were forward, 5′-ATGGGTGTGAACCACGAGAA-3′ and reverse, 5′-AGTTGCTGTTGAAGTCGCAG-3′. The PCR products were subjected to electrophoresis on 1% agarose gels, and the signal was visualized with Fluor-S Multiimager (Bio-Rad, Hercules, CA). Quantitative data were obtained using the Quantity One software (Bio-Rad). For quantitative RT-PCR, total RNA was reverse-transcribed into cDNA with avian myeloblastosis virus reverse transcriptase using random hexamer primers (Roche, Indianapolis, IN). Primers and probes for human Btk were purchased as predeveloped TaqMan assays (Assays-on-Demand; Applied Biosystems, Foster City, CA). 18S rRNA was used as an endogenous control. Quantitative RT-PCR was performed as previously described.31
Immunoprecipitation and immunoblotting
Cells were routinely analyzed 48 hours after transfection. Immunoprecipitation and immunoblotting were performed essentially as previously described.29
Hydrodynamic transfection
Hydrodynamic transfections of plasmids in Ringer solution were carried out as previously described.32,33 Briefly, 8% vol/wt Ringer solution containing plasmids and oligonucleotide decoys was introduced by tail vein injection over a period of 5 seconds to inbred NMRI mice. Live, anesthetized mice were imaged for 10 seconds to 5 minutes using an intensified CCD camera (IVIS imaging system, Caliper Life Sciences, Hopkinton, MA).
Ethics permission
All animal research was approved by the Local Committee for Animal Ethics in Stockholm, Sweden, and performed in accordance with the ethics permission.
Bioinformatic tools used for identification of transcription factor (TF)–binding sites in the Btk promoter
In silico analysis was made on the Btk promoter and the enhancer regions. A sequence corresponding to 1500-bp upstream of the Btk transcription start site was analyzed. The computational scan for transcription factor–binding sites (TFBSs) was performed using TRANSFAC Database 7.0-public (http://www.biobase-international.com),34 and P-Match software.35 A matrix (TFP60pm) was chosen and 3 scans were performed using alternative parameter settings to minimize the false negative or positive rates with regard to the Btk promoter sequence.
Electrophoretic mobility shift assay (EMSA)
A20 cells were washed twice with ice-cold phosphate-buffered saline. Following cell lysis, crude nuclear protein was obtained using a cytosolic and nuclear protein extraction kit (Pierce). EMSA was performed using LightShift chemiluminescent EMSA kit (Pierce). The oligonucleotide probes were as follows: for NF-κB putative site 1, the wild-type oligonucleotide sequence (Wt-1) was 5′-TCCACAGAAAATGGGGAAGGGCACAAGTAT-3′, and the mutant sequence (Mu-1) was 5′-TCCACAGAAAATGTTTAAGGGCACAAGTAT-3′. For NF-κB putative site 2, the wild-type oligonucleotide sequences (Wt-2) was 5′-CTGCATTTCCTAGGAGAATCCCTGGGGGAA-3′, and the mutant oligonucleotide (Mu-2) was 5′-CTGCATTTCCTATTTTAATCCCTGGGGGAA-3′. DNA-protein–binding was performed at room temperature for 20 minutes in a final volume of 20 μL containing binding buffer (10 mM Tris, pH 7.5, 50 mM KCl, 1 mM dithiothreitol), 2.5% (vol/vol) glycerol, 5 mM MgCl2, 1 μg poly(dI-dC), 0.05% (vol/vol) Nonidet P-40, 8 pmol double-stranded biotinylated probe, and 10 μg nuclear extract. The DNA-protein complexes were separated by 6% polyacrylamide gel electrophoresis (PAGE) in 0.5 × TBE at 100 V at 4°C for 1.5 hours. DNA-protein complexes in the gel were transferred to Hybond N nylon membrane (GE Healthcare, Little Chalfont, United Kingdom) by electroblotting with 0.5 × TBE at 100 V for 40 minutes. DNA-protein complexes were fixed to the membrane by a UV cross-linker and detected by a nonradioactive nucleic acid detection kit (Pierce). For the competition assay, 100-fold excess cold double-stranded DNAs were included in the binding reaction. Anti-p65 antibody or anti-p50 antibody (1 μL) was preincubated in the binding reaction for 10 minutes before the probe addition.
Chromatin immunoprecipitation assay
ChIP was performed essentially as reported.36 Cross-linking of protein-DNA complex in vivo was achieved by incubating cells in 1% formaldehyde solution for 10 minutes at room temperature. The cross-linking reaction was quenched by 125 mM glycine (final concentration) for 5 minutes at room temperature. Cells were washed twice with cold PBS, lysed, and subjected to sonication at 4°C using the UP200S Ultraschallprozessor sonicator (Buddeberg, Berlin, Germany). The sonicated lysates were first precleared by adding 50 μL protein A-agarose beads and rotated for 1 hour at 4°C. Subsequently, lysates were subjected to immunoprecipitation by adding 5 μL polyclonal anti-p50, anti-p65, anti-PU.1, or rabbit normal Ig, respectively. Samples were rotated overnight at 4°C; 50 μL protein A-agarose beads was added to each sample followed by rotation for another 2 hours. The proteins were removed from DNA by digesting with 10 μg/mL proteinase K at 65°C for 30 minutes. The DNA was further purified by QIAquick PCR purification kit (Qiagen) and eluted in 50 μL sterile water. Eluted DNA (2 μL) was used for the PCR assay to detect DNA physically associated with the immunoprecipitated proteins. The primers for the NF-κB–binding sequence were forward primer, 5′-TATGGCAGTGCCCGAGGTGT-3′, and reverse primer, 5′-GACAAGACTTCCCCGTGCTG-3′. Primers for PU.1-binding sequence were forward primer, 5′-TTGATGACCTGTCTGCTCAGG-3′ and reverse primer, 5′-TGAG-ATGCCAGGACTTGGAAG-3′. Values obtained from immunoprecipitated samples were normalized to input samples.
Results
Proteasome inhibitors down-regulate Btk expression both in B-cell lines and primary B cells
The majority of intracellular proteins in eukaryotic cells are degraded through the ubiquitin-proteasome pathway. To determine whether the 26S proteasome also regulates Btk, we treated the mouse B-lymphocyte cell line A20 with the proteasome inhibitor MG132. Surprisingly, treatment of cells with this compound led to a dramatic reduction in the steady-state levels of Btk, while expression of MAP kinases was not affected (Figure 1A). Similar results were obtained in 2 other human cell lines, the B-cell line Ramos and the pre-B-cell line Nalm6 (Figure 1B and data not shown). Expression of the Src family kinase member, Lyn, remained constant, while, as expected, the steady-state levels of p53 increased following treatment with the proteasome inhibitor. Likewise, in primary B cells, upon MG132 treatment, the steady-state level of Btk was reduced after 10 hours, and almost abolished after 15 hours (Figure 1C). Altogether, our data indicate that proteasome inhibitors reduce Btk expression both in B-cell lines and primary B cells.
Proteasome inhibitors down-regulate Btk expression both in B-cell lines and primary B cells. (A) A20 cells were treated with different concentrations of MG132 for 16 hours. Cells were lysed and subjected to Western blot analysis. (B) Nalm6 pre-B cells were treated with ALLN (150 μM), MG-132 (10 μM), lactacystin (10 μM), or DMSO alone for 16 hours. Cells were lysed and subjected to Western blot analysis. (C) Purified splenic B cells from C57BL/6 wild-type mice were cultured in the absence or presence of 10 μM MG132 for 5, 10, and 15 hours as indicated in the figure. Total cell lysates were processed for immunoblotting analysis for Btk and actin.
Proteasome inhibitors down-regulate Btk expression both in B-cell lines and primary B cells. (A) A20 cells were treated with different concentrations of MG132 for 16 hours. Cells were lysed and subjected to Western blot analysis. (B) Nalm6 pre-B cells were treated with ALLN (150 μM), MG-132 (10 μM), lactacystin (10 μM), or DMSO alone for 16 hours. Cells were lysed and subjected to Western blot analysis. (C) Purified splenic B cells from C57BL/6 wild-type mice were cultured in the absence or presence of 10 μM MG132 for 5, 10, and 15 hours as indicated in the figure. Total cell lysates were processed for immunoblotting analysis for Btk and actin.
Proteasome inhibitors reduce expression of other Tec family kinases
Since our results show that proteasome inhibitors reduce steady-state levels of Btk, we sought to investigate whether these drugs also display similar effect toward other members of Tec family kinases. Jurkat, Huvec, and A20 cells were treated with different concentrations of MG132. These cell lines express Itk, Bmx, and Tec, respectively. Similar to our findings on Btk, expression of Itk, Bmx, and Tec was compromised following treatment of cells with the drug (Figure 2). These results strongly suggest that there might be a common mechanism responsible for regulating the expression of these kinases.
Proteasome inhibitors reduce the expression of Tec family kinases Btk, Itk, Bmx, and Tec. K562 (A), Jurkat (B), Huvec (C), and A20 (D) cells were treated with different concentrations (μM) of MG132 as indicated for 16 hours. Cell lysates were subjected to Western blot analysis as indicated.
Proteasome inhibitors reduce the expression of Tec family kinases Btk, Itk, Bmx, and Tec. K562 (A), Jurkat (B), Huvec (C), and A20 (D) cells were treated with different concentrations (μM) of MG132 as indicated for 16 hours. Cell lysates were subjected to Western blot analysis as indicated.
Promoter-dependent inhibition of Btk expression by proteasome inhibitors
To get a deeper insight into the role of proteasomes in the regulation of Btk, we transiently transfected expression vectors encoding Btk-GFP into 2 widely used cell lines, COS-7 and 293T. In contrast to the situation in B cells, treatment of the cell lines with MG132 led to a dramatic accumulation of the protein (Figure 3A). To further determine whether these observations were due to transcriptional or translational events, we used a Btk-deficient chicken B-cell line stably expressing human Btk under the control of the strong chicken β-actin promoter (B41-13 cells). In this particular system, the Btk gene is functionally expressed from the native or a foreign promoter, respectively, but within the same cellular environment. Using species-specific anti-Btk antibodies, we found that the steady-state level of Btk was slightly increased in the B41-13 cell line by the treatment with MG132. Conversely, treatment of DT40 cells (wild-type) led to a significant decrease of the protein (Figure 3B), suggesting promoter-related transcriptional effects as the primary underlying mechanism.
Effects of proteasome inhibitors on Btk expression in heterologous cells and in chicken B-cell lines. (A) 293T and COS-7 cells were transiently transfected with Btk-GFP. Cells were cultured in the presence or absence of 10 μM MG132 for 16 hours, and total cell lysates were subjected to Western blot analysis. (B) B41-13 (Btk-defective chicken B cells reconstituted with human Btk under the control of the chicken β-actin promoter) and DT40 cells were treated with MG132 as indicated, and cell extracts were subjected to immunoblotting analysis (the blot from B41-13 cell extracts was decorated with polyclonal antihuman Btk antibody, whereas polyclonal antichicken Btk antibody was used in the blot of the DT40 lysates).
Effects of proteasome inhibitors on Btk expression in heterologous cells and in chicken B-cell lines. (A) 293T and COS-7 cells were transiently transfected with Btk-GFP. Cells were cultured in the presence or absence of 10 μM MG132 for 16 hours, and total cell lysates were subjected to Western blot analysis. (B) B41-13 (Btk-defective chicken B cells reconstituted with human Btk under the control of the chicken β-actin promoter) and DT40 cells were treated with MG132 as indicated, and cell extracts were subjected to immunoblotting analysis (the blot from B41-13 cell extracts was decorated with polyclonal antihuman Btk antibody, whereas polyclonal antichicken Btk antibody was used in the blot of the DT40 lysates).
Thus, with heterologous promoters, β-actin promoter in chicken cells or CMV promoter in COS-7 or 293T cells, Btk was not subject to negative regulation by proteasome inhibitors, invalidating the role of translation in the down-regulation of Btk. Conversely, steady-state levels of Btk increased following treatment of the transfected cells with MG132 or other proteasome inhibitors (Figure 3A and data not shown), indicating that Btk might be degraded via the 26S proteasome pathway.
NF-κB signaling regulates Btk transcription
To confirm that proteasome inhibitors reduce Btk transcription, we extracted RNA from A20 cells previously treated with MG132. Steady-state levels of Btk mRNA were severely reduced (Figure 4A). Furthermore, identical results were obtained in Ramos and Nalm6 cells (Figure 4B and data not shown). To get a deeper insight into this, we subsequently used bioinformatics tools to scan the Btk promoter to identify possible binding elements for transcription factors. We were able to identify 2 putative NF-κB–binding sites, which localize to the region −600-bp to −800-bp upstream of the transcription initiation site (Figure 4C). To further determine whether these sites are functionally required, we generated 1000-bp and 500-bp amplicons from genomic DNA, and subcloned them into reporter plasmids. We found that in A20 cells expression from the longer promoter construct (1000-Btk) was more robust than from the short one (500-Btk), indicating possible involvement of the NF-κB elements. In addition, treatment with MG132 resulted in a dramatic reduction of the transcription activity from the 1000-Btk promoter construct. In contrast, transcription from the 500-Btk amplicon slightly increased following treatment with the proteasome inhibitor (Figure 4D). This suggests that the proteasome inhibitor–responsive elements are located inside the region between 1000-bp and 500-bp upstream of the start site. Since global proteasome inhibition leads also to inactivation of NF-κB signaling, we wanted to find out whether specific inhibition of NF-κB could block transcription of Btk. Indeed, treatment with the highly specific NF-κB inhibitor Bay 11-7085 reduced Btk expression dramatically (Figure 4E). In addition, we show that the clinically approved proteasome inhibitor, bortezomib, has a similar effect on Btk transcription. Taken together, these results indicate that NF-κB transcriptionally regulates the expression of Btk.
Proteasome and/or NF-κB inhibitors regulate Btk transcription. (A) A20 cells were treated with or without MG132 for 16 hours and total RNA was isolated and subjected to RT-PCR. Relative amount of Btk mRNA levels is demonstrated at the bottom of the figure. Data are representative of 3 independent experiments. (B) Ramos cells were treated with or without MG132 for 16 hours. The amount of Btk mRNA was evaluated by quantitative RT-PCR (triplicate samples were run). Each sample was normalized using 18S rRNA as a control. (C) Schematic representation showing the structure of the Btk promoter. Depicted here are some transcription factors and coactivators (OCT1, PU.1, and sp1/sp3) that have been demonstrated to bind to the Btk promoter. Btk promoter reporter constructs (500-Btk and 1000-Btk) and 2 putative NF-κB–binding sites are also indicated. (D) The 500-Btk and 1000-Btk promoter constructs were introduced into A20 cells, and cells were treated with or without MG132 for 16 hours. Luciferase activity was measured as described in “Methods”; relative levels of luciferase activity are shown. Data are representative of 3 independent experiments. (E) A20 cells were treated with bortezomib (20 nM) or Bay 11-7085 (5 μM) for 16 hours. Cell lysates were subjected to Western blot analysis as indicated. (F) NMRI mice were transfected with 20 μg of the 1000-Btk promoter reporter construct using the hydrodynamic procedure. In addition, the mouse in the middle received 1 mg/kg bortezomib and the mouse to the right received 1 mg/kg LPS. In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments.
Proteasome and/or NF-κB inhibitors regulate Btk transcription. (A) A20 cells were treated with or without MG132 for 16 hours and total RNA was isolated and subjected to RT-PCR. Relative amount of Btk mRNA levels is demonstrated at the bottom of the figure. Data are representative of 3 independent experiments. (B) Ramos cells were treated with or without MG132 for 16 hours. The amount of Btk mRNA was evaluated by quantitative RT-PCR (triplicate samples were run). Each sample was normalized using 18S rRNA as a control. (C) Schematic representation showing the structure of the Btk promoter. Depicted here are some transcription factors and coactivators (OCT1, PU.1, and sp1/sp3) that have been demonstrated to bind to the Btk promoter. Btk promoter reporter constructs (500-Btk and 1000-Btk) and 2 putative NF-κB–binding sites are also indicated. (D) The 500-Btk and 1000-Btk promoter constructs were introduced into A20 cells, and cells were treated with or without MG132 for 16 hours. Luciferase activity was measured as described in “Methods”; relative levels of luciferase activity are shown. Data are representative of 3 independent experiments. (E) A20 cells were treated with bortezomib (20 nM) or Bay 11-7085 (5 μM) for 16 hours. Cell lysates were subjected to Western blot analysis as indicated. (F) NMRI mice were transfected with 20 μg of the 1000-Btk promoter reporter construct using the hydrodynamic procedure. In addition, the mouse in the middle received 1 mg/kg bortezomib and the mouse to the right received 1 mg/kg LPS. In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments.
In vivo analysis of the Btk promoter
To determine whether NF-κB induces Btk expression in vivo, we used the hydrodynamic infusion technique.32,33 This method is robust in its ability to deliver foreign DNA into liver. We introduced the Btk reporter construct (1000-Btk) into the livers of mice, and the luciferase-derived signal in live mice was monitored using bioluminescence imaging. Figure 4F shows that Btk reporter was expressed in the liver. The injection of the only clinically approved proteasome inhibitor, bortezomib, into these mice led to a dramatic decrease in the steady-state levels of the luciferase reporter. This result is consistent with the in vitro data in the cell lines. Moreover, LPS treatment increased the Btk reporter expression up to 4.6 times over the control, which is also compatible with the in vitro data, implicating NF-κB signaling in the transcription of the Btk gene. Taken together, these findings suggest that NF-κB signaling is critical for the transcription of Btk. To the best of our knowledge, this is the first time a signaling pathway has been addressed using hydrodynamic transfection.
Btk expression correlates with nuclear NF-κB levels
It is known that in resting cells the transcription factor NF-κB is predominantly cytosolic and sequestered by the inhibitor protein I-κB. Following activation of the NF-κB signaling pathway, I-κB is phosphorylated and degraded by the ubiquitin proteasome pathway, leading to the release and nuclear translocation of the NF-κB dimer. Proteasome inhibitors inactivate NF-κB signaling by stabilizing the inhibitor I-κB.37-39 Consequently, as shown in Figure 4A,B, proteasome inhibitors induced transcriptional down-regulation of the Btk gene. To further determine whether this proteasome-dependent transcription regulation requires de novo protein synthesis, A20 cells were pretreated with cycloheximide for 1 hour followed by incubation with MG132 or epoxomicine for 16 hours. We found that cycloheximide blocked the down-regulation of Btk expression induced by MG132 or epoxomicine, while under these conditions cycloheximide alone did not affect Btk expression (Figure 5A). As shown in the figure, the augmented global protein ubiquitination level clearly shows the activity of MG132 and epoxomicine. This effect was reversed by cycloheximide, and all these reagents did not affect the control proteins ERK1 and ERK2. This indicates that a labile factor, presumably I-κB, is involved in the proteasome-dependent down-regulation of Btk.
Proteasome inhibitors suppress Btk transcription, a phenomenon requiring de novo protein synthesis. (A) A20 cells were treated with CHX (10 μg/mL) an hour prior to the treatment with epoxomicin (10 nM) or MG132 as indicated. Cell lysates were subjected to Western blot analysis. Total protein ubiquitin level was used for assessing the activity of the proteasome inhibitors, and ERK1 and ERK2 served as internal controls. An unknown 50-kD protein also served as loading control. (B) A20 cells were treated with CHX and MG132 as in panel A. Cytosolic and nuclear proteins were obtained using an extraction kit from Pierce, and proteins were processed for Western blot analysis as indicated in the figure. (According to the company's instruction, the cytosolic proteins were finally dissolved in 211 μL buffer, whereas nuclear proteins were dissolved in 100 μL buffer.)
Proteasome inhibitors suppress Btk transcription, a phenomenon requiring de novo protein synthesis. (A) A20 cells were treated with CHX (10 μg/mL) an hour prior to the treatment with epoxomicin (10 nM) or MG132 as indicated. Cell lysates were subjected to Western blot analysis. Total protein ubiquitin level was used for assessing the activity of the proteasome inhibitors, and ERK1 and ERK2 served as internal controls. An unknown 50-kD protein also served as loading control. (B) A20 cells were treated with CHX and MG132 as in panel A. Cytosolic and nuclear proteins were obtained using an extraction kit from Pierce, and proteins were processed for Western blot analysis as indicated in the figure. (According to the company's instruction, the cytosolic proteins were finally dissolved in 211 μL buffer, whereas nuclear proteins were dissolved in 100 μL buffer.)
To investigate whether this highly unstable, elusive factor is critical for regulating subcellular localization of NF-κB, we treated cells with MG132 in the presence or absence of cycloheximide and analyzed the steady-state levels of NF-κB in the cytosolic and nuclear fractions. Our data show that the p65/RelA subunit of NF-κB accumulates in the nucleus following cycloheximide treatment of cells (Figure 5B). Overall, these findings demonstrate that NF-κB levels inside the nucleus correlate with the degree of expression of Btk, and might be directly responsible for the transcriptional regulation of Btk.
NF-κB binds directly to the Btk promoter
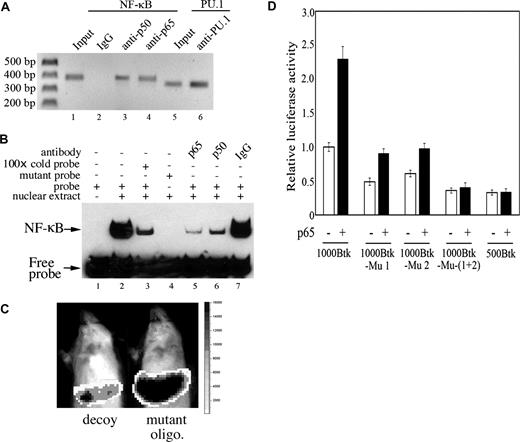
Since the computational scan for transcription factor–binding sites identified 2 putative NF-κB–binding sites in the Btk promoter (Figure 4C), we decided to determine their functional relevance. Using the chromatin immunoprecipitation (ChIP) assay, we found that the NF-κB subunits, p65 and p50, readily associate with the Btk promoter (Figure 6A lanes 3,4).
NF-κB binds directly to the Btk promoter and induces Btk transcription. (A) The chromatin of A20 cells was cross-linked, sheared, and immunoprecipitated with the indicated antibodies. Input and immunoprecipitated DNA was purified and used as template in PCR with primers specific for the Btk promoter (region from −881 to −500 that contain the 2 putative NF-κB sites). Primers for PU.1 (region from −261 to +91, which contains the PU.1 site) were used as positive control; rabbit normal IgG in lane 2 serves as negative control. (B) Nuclear extracts from A20 cells were prepared and DNA-protein–binding activity was analyzed by EMSA. Labeled oligonucleotide probe (lanes 1-4 and 5-7) for NF-κB site 1 (Figure 4C) is described in “Methods.” A labeled probe was incubated without (lane 1, negative control) or with (lanes 2-7) nuclear protein. One hundred–fold molar excess of unlabeled oligonucleotide (cold probe) was used as competitor (lane 3). Anti-p65 antibody, anti-p50 antibody, and rabbit normal IgG are present in lanes 5, 6, and 7, respectively. The probe was replaced by the mutant probe in lane 4. (C) NMRI mice were transfected with 30 μg 1000-Btk (Btk promoter reporter construct) using the hydrodynamic procedure. In addition, the mouse to the left received 7 nmol mixed oligonucleotide decoys (Wt-1 + Wt-2, oligonucleotides corresponding to the NF-κB–binding sites 1 and 2 elements in Btk promoter; details in “Methods”). The mouse to the right received 7 nmol mixed mutant oligonucleotides (Mu-1 + Mu-2). In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments. (D) The 500-Btk, and the 1000-Btk and its mutant promoter reporter constructs (1000-Btk-Mu 1, 1000-Btk-Mu 2, and 1000-Btk-Mu-(1 + 2)) were cotransfected with p65/RelA plasmid into A20 cells. Forty-eight hours later, luciferase activity was measured as described in “Methods”; relative levels of luciferase activity are shown. Data are representative of 3 independent experiments.
NF-κB binds directly to the Btk promoter and induces Btk transcription. (A) The chromatin of A20 cells was cross-linked, sheared, and immunoprecipitated with the indicated antibodies. Input and immunoprecipitated DNA was purified and used as template in PCR with primers specific for the Btk promoter (region from −881 to −500 that contain the 2 putative NF-κB sites). Primers for PU.1 (region from −261 to +91, which contains the PU.1 site) were used as positive control; rabbit normal IgG in lane 2 serves as negative control. (B) Nuclear extracts from A20 cells were prepared and DNA-protein–binding activity was analyzed by EMSA. Labeled oligonucleotide probe (lanes 1-4 and 5-7) for NF-κB site 1 (Figure 4C) is described in “Methods.” A labeled probe was incubated without (lane 1, negative control) or with (lanes 2-7) nuclear protein. One hundred–fold molar excess of unlabeled oligonucleotide (cold probe) was used as competitor (lane 3). Anti-p65 antibody, anti-p50 antibody, and rabbit normal IgG are present in lanes 5, 6, and 7, respectively. The probe was replaced by the mutant probe in lane 4. (C) NMRI mice were transfected with 30 μg 1000-Btk (Btk promoter reporter construct) using the hydrodynamic procedure. In addition, the mouse to the left received 7 nmol mixed oligonucleotide decoys (Wt-1 + Wt-2, oligonucleotides corresponding to the NF-κB–binding sites 1 and 2 elements in Btk promoter; details in “Methods”). The mouse to the right received 7 nmol mixed mutant oligonucleotides (Mu-1 + Mu-2). In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments. (D) The 500-Btk, and the 1000-Btk and its mutant promoter reporter constructs (1000-Btk-Mu 1, 1000-Btk-Mu 2, and 1000-Btk-Mu-(1 + 2)) were cotransfected with p65/RelA plasmid into A20 cells. Forty-eight hours later, luciferase activity was measured as described in “Methods”; relative levels of luciferase activity are shown. Data are representative of 3 independent experiments.
Having identified 2 NF-κB–binding sites in the Btk promoter (Figure 6A), it was desirable to determine whether both of these sites were active. To this end, we used the electrophoretic mobility shift assay (EMSA). We found that both sites are indeed used and are bona fide NF-κB–binding sites (Figure 6B and data not shown). To investigate whether p50 or p65 is involved in binding to these elements, we added antibodies against both subunits. Although a clear and distinct supershift was not obtained with these antibodies, binding of the labeled probe to the nuclear extract specifically diminished, suggesting that the antiserum generated immunocomplexes rather than supershifts of oligonucleotides bound to p50/p65. These results clearly demonstrate that NF-κB binds, both in vivo and in vitro, to the Btk promoter.
In vivo inhibition of NF-κB–dependent transcription of Btk by oligonucleotide decoys
Since the regulatory region of the Btk gene harbors 2 classical NF-κB–binding sites, it was of considerable interest to assess their in vivo potential as transcriptional enhancers of the promoter. To this end, we decided to investigate whether oligonucleotides corresponding to these putative NF-κB–binding elements could be used as molecular decoys for inhibiting transcription of the Btk promoter. We therefore used the powerful hydrodynamic delivery system as a tool for introducing the 1000-Btk (Btk reporter construct) together with the oligonucleotides into the liver of mice. Interestingly, expression of the luciferase reporter gene was found to be severely abrogated following hydrodynamic delivery of oligonucleotides corresponding to the NF-κB–binding elements (Figure 6C). To our knowledge, this is the first report of its kind showing the use of oligos as decoys for inhibiting transcription in the liver using hydrodynamics.
NF-κB up-regulates Btk gene expression
To address whether NF-κB itself maintains a positive regulatory role in the transcription of Btk, an expression vector encoding p65/RelA was introduced together with the 1000-Btk promoter construct into the B-cell line A20. As shown in Figure 6D, there was significant induction of luciferase activity (up to 2.3-fold) in cells overexpressing p65/RelA. In contrast, transcription from the truncated 500-Btk promoter construct, which lacks the putative NF-κB–binding elements, remained unchanged. To further investigate whether one or both sites are functionally utilized, we used promoter constructs that harbor mutations in one or both of the NF-κB–binding elements (1000-Btk mutants Mu 1, Mu 2, and Mu (1 + 2)) and expressed them together with p65/RelA in A20 cells. Whereas mutation of either of these sites compromises the activity of the Btk promoter, mutation of both sites resulted in severe reduction of the reporter gene activity (Figure 6D and not shown). Altogether, our results demonstrate that p65/RelA induces transcription of Btk. Moreover, we herein present direct evidence that the 2 putative NF-κB–binding sites are functionally competent.
Btk induces its own promoter via NF-κB signaling
Previous work on cell lines has shown that Btk is essential for the activation of NF-κB, and that the nuclear translocation of NF-κB is impaired in Btk-deficient B cells following BCR stimulation.13,14,37 To investigate whether Btk stimulates in vivo the NF-κB signaling pathway, mice were hydrodynamically transfected with a luciferase reporter construct encoding a minimal eukaryotic promoter with NF-κB–responsive elements. Three days after injection, mice received a construct encoding constitutively active Btk (E41K-Btk) and 24 hours later luciferase expression was recorded. As depicted in Figure 7A (middle panel), Btk expression was found to strongly activate the NF-κB signaling pathway. Similarly, LPS treatment of the mice led to a robust activation of NF-κB, which was completely abrogated by the proteasome inhibitor bortezomib (Figure 7A bottom panel). To explore whether Btk itself is able to induce transcription from its own promoter, A20 cells were cotransfected with 1000-Btk reporter construct and E41K-Btk. We found that, in the presence of E41K-Btk, transcription of Btk increased 7-fold (Figure 7B), suggesting that Btk can induce its own promoter. This indicates that Btk uses a positive feedback loop to activate its own transcription via NF-κB.
Btk induces its own promoter via NF-κB. (A) NMRI mice were transfected with 1 μg pNF-κB-Luc (NF-κB reporter construct) using the hydrodynamic procedure. At days 4, 5, and 8, mice received a second transfection with 10 μg E41K-Btk and/or were treated with LPS or bortezomib, respectively, as indicated in the figure. In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments. (B) A20 cells were transfected with the 1000-Btk reporter construct with or without a constitutively active form of Btk (E41K-Btk). Luciferase activity was measured and relative levels of luciferase activity are shown. Data are representative of 3 independent experiments. (C) Schematic diagram showing the Btk→NF-κB→Btk signaling module. Following BCR stimulation, Btk together with other components of the BCR signalsome activate the transcription factor NF-κB. NF-κB translocates into the nucleus, binds to the Btk promoter, and induces transcription. The newly synthesized Btk forms a positive feedback loop to activate the Btk→NF-κB→Btk signaling pathway. In addition, NF-κB binds to its own promoter to positively autoregulate its transcription.15,40
Btk induces its own promoter via NF-κB. (A) NMRI mice were transfected with 1 μg pNF-κB-Luc (NF-κB reporter construct) using the hydrodynamic procedure. At days 4, 5, and 8, mice received a second transfection with 10 μg E41K-Btk and/or were treated with LPS or bortezomib, respectively, as indicated in the figure. In vivo biophotonic imaging was performed using the IVIS imaging system as described in “Methods.” Data are representative of 3 independent experiments. (B) A20 cells were transfected with the 1000-Btk reporter construct with or without a constitutively active form of Btk (E41K-Btk). Luciferase activity was measured and relative levels of luciferase activity are shown. Data are representative of 3 independent experiments. (C) Schematic diagram showing the Btk→NF-κB→Btk signaling module. Following BCR stimulation, Btk together with other components of the BCR signalsome activate the transcription factor NF-κB. NF-κB translocates into the nucleus, binds to the Btk promoter, and induces transcription. The newly synthesized Btk forms a positive feedback loop to activate the Btk→NF-κB→Btk signaling pathway. In addition, NF-κB binds to its own promoter to positively autoregulate its transcription.15,40
Discussion
The major finding of this study is the discovery that NF-κB is required for adequate expression of the Btk gene, and that proteasome and NF-κB inhibitors decrease Btk transcription. In addition, we show that p65/Rel A can directly activate the Btk promoter. To our knowledge, this is the first report showing NF-κB regulation of a nonreceptor tyrosine kinase promoter.
Although the Btk promoter has been analyzed quite extensively with respect to its binding of transcription factors,22-25,41-43 the presence of functional NF-κB–binding elements has not been reported, presumably because most published studies focused on the core promoter region (ie, within −300 of the start site). In the present work, we demonstrate that 2 functionally active NF-κB–binding motifs are located further upstream.
NF-κB is important for the survival of developing lymphocytes and is essential for maturation of B cells in the spleen.37,44,45 Moreover, it is well known that Btk is a critical component in the signalsome that regulates B-cell proliferation and differentiation. Previous studies have shown that NF-κB is one of the downstream signaling molecules of Btk.12-14 Apart from cell line experiments, we have used an oligonucleotide-based molecular decoy approach for studying gene expression in the liver of live mice. This, together with our finding regarding the LPS-stimulated transcription of the Btk promoter, open up new avenues for the use of hydrodynamic gene delivery in addressing questions related to in vivo cellular signaling and transcriptional regulation. Notably, an important application of this technology is to use it in investigating molecular signaling pathways in hepatocytes.
The ubiquitin proteasome degradation pathway plays critical roles in many cellular processes,46 and there is increasing evidence that the ubiquitin proteasome degradation pathway is also involved in regulating transcription. We found that proteasome inhibitors differentially affect steady-state levels of Btk expressed from endogenous versus heterologous promoters. In primary B cells, as well as in B-cell lines, Btk transcription was markedly reduced by the proteasome inhibitors (Figure 1). Significantly, using the hydrodynamic infusion technique, we replicated this in vitro data in mice and found that the proteasome inhibitor, bortezomib, reduced Btk expression. Moreover, expression of other members of Tec family kinases was also compromised following treatment with such inhibitors (Figure 2). Altogether, these results suggest that there is a common mechanism regulating expression of the Tec family kinases.
The fact that ectopically expressed Btk increased when cells were treated with MG132 or other proteasome inhibitors implicates transcription, rather than translation, as the target. One potential candidate is the transcription factor NF-κB, a master regulator of genes involved in immunity and inflammation. To directly determine whether NF-κB itself was the main culprit responsible for the observed phenotype on the proteasome-treated B-cell lines, we used a highly specific NF-κB inhibitor, BAY-117085. Btk levels dropped sharply when cells were treated with this drug, suggesting that NF-κB was indeed the key factor responsible for this phenomenon.
This is a very interesting relationship, in light of the fact that Btk and NF-κB have been shown to be functionally intertwined. First, both Btk and NF-κB are indispensable for B-cell proliferation, differentiation, and survival. Second, the BCR-dependent NF-κB signaling pathway has been shown to be abrogated in B lymphocytes that are deficient in Btk. In the case of Btk, the same mutation in the Btk gene is known to be responsible for XLA in humans and xid in mice.47 However, the ensuing primary immunodeficiency is more severe in humans than in mice, and, while redundancy of the Tec kinase in mice has been demonstrated,48 the molecular basis of this species difference remains to be established. In the present study, we demonstrate that NF-κB (p50/p65) directly regulates transcription of the Btk gene. Furthermore, data from our and other laboratories show that NF-κB signaling requires functional Btk.12-15 It is conceivable that NF-κB signaling could differ between humans and mice, contributing to the pathology of Btk deficiency.
NF-κB is pivotal for the induction of host-defense genes during acute pathogenic threats. In addition, NF-κB signaling is critical for lymphocyte proliferation, survival, and differentiation. Nonetheless, these cellular functions of NF-κB can be subverted in a variety of diseases. For example, abnormal activation of NF-κB has been shown to be involved in the survival, development, and progression of tumors.49-54 In addition, the widespread involvement of NF-κB in cancer, and inflammatory and autoimmune diseases has made it an important pharmacological target. In this study, we found that not only the experimental drugs used as NF-κB inhibitors (MG132 and Bay 11-7085) but also the clinically approved drug, bortezomib, inhibited Btk expression. Bortezomib is routinely used in treating B-cell tumors, such as multiple myeloma.55
Proteasome dysfunction leads to the accumulation of the NF-κB inhibitor I-κB and subsequent inactivation of the NF-κB signaling pathway. To determine whether this requires de novo protein synthesis, we treated cells with cycloheximide prior to the proteasome inhibitors. We found that the effect of proteasome inhibitors on Btk was reversed following treatment with the protein synthesis inhibitor. This indicates that a labile factor with a very short half-life is most likely involved in the proteasome-dependent down-regulation of Btk. Of note, a known target of canonical NF-κB signaling is the gene encoding the IκB proteins. Accordingly, following NF-κB activation, transcription of the I-κB inhibitor protein increases, leading to its accumulation. Minutes later, the newly synthesized I-κB translocates to the nucleus to fetch NF-κB dimer and sequester it in the cytoplasm. In B cells, I-κB has a very short half-life, extending to only 40 minutes.56 We therefore believe the main reason that cycloheximide reversed proteasome-dependent down-regulation of Btk was the depletion of I-κB. Thus, without de novo protein synthesis, the remaining pool of I-κB is quickly depleted, leading to the accumulation of nuclear NF-κB. Recently, it has been shown that cycloheximide not only enhanced the nuclear accumulation of NF-κB, but also induced its DNA-binding activity.57 Our data on the cycloheximide-induced effect on I-κB could also explain the fact that proteasome inhibitors decreased transcription of the elastin gene, and that cycloheximide treatment restored elastin mRNA levels.58
Moreover, we demonstrate that Btk can induce its own promoter via NF-κB (Figure 7B). Based on the results of the current study and those of others, we propose the following model. Upon BCR stimulation, Btk translocates to lipid rafts (caveolae) to join a multiprotein complex structure, the BCR signalsome. In this dynamic milieu, Btk becomes phosphorylated and activates, among other downstream signaling proteins, the transcription factor NF-κB. Subsequently, NF-κB binds to regulatory elements in the Btk promoter inducing transcription. Next, newly synthesized Btk further stimulates NF-κB signaling forming a positive feedback loop (Figure 7C). Interestingly, NF-κB also positively autoregulates its own transcription.15,40 Together this results in a positive, dual feedback loop for Btk, involving multiple levels of interdependencies. To control the positive feedback, Btk is subject to negative regulation by several proteins including PKC, caveolin-1, and Pin1.29,59,60
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Cancer Fund, the Wallenberg Foundation, the Swedish Science Council, the Swedish Hemophilia Society, the Stockholm County Council (research grant ALF-projektmedel medicin), and the European Union Grant EURO-POLICY-PID.
Authorship
Contribution: L.Y. designed and performed the majority of the research, analyzed the data, and wrote the paper; A.J.M. designed the research, analyzed the data, and wrote and revised the paper; O.E.S. and H.J.A. performed animal experiments including hydrodynamic transfection and developed the in vivo signaling procedure as well as the hydrodynamic delivery of decoy oligonucleotides; L.V. and K.E.M.B. performed some of the experiments; B.B. contributed vital reagents; B.F.N. performed the promoter analysis and some of the experiments; C.I.E.S. conceived the project, provided supervision throughout, interpreted data, and helped with writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Liang Yu, Department of Laboratory Medicine, Clinical Research Center, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-141 86, Stockholm, Sweden; e-mail: liang.yu@ki.se; and C. I. Edvard Smith, Department of Laboratory Medicine, Clinical Research Center, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-141 86, Stockholm, Sweden; e-mail: edvard.smith@ki.se.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal