To the editor:
Patients undergoing solid organ transplantations carry a significant risk of developing posttransplantation lymphoproliferative disorders (PTLDs).1 Only a minority of PTLD cases are of T-cell origin, accounting for 6% to 8% of cases.2,3 T-cell PTLDs tend to occur later in the posttransplantation course and are generally resistant to therapy. In one series, the median survival was 5 weeks.4 Treatment of T-cell PTLDs includes reduction of immunosuppression and various chemotherapy regimens, neither of which have been successful.2,4
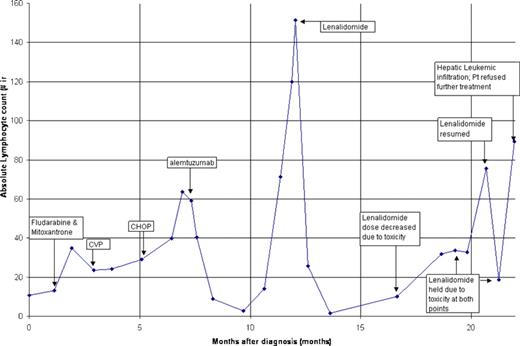
We describe a 62-year-old female patient who developed T-cell PTLD 7 years after receiving a cadaveric renal transplant for autosomal dominant polycystic kidney disease. Her immunosuppressants included cyclosporine and prednisone. She presented with excessive fatigue and lymphocytosis. Her initial complete blood count (CBC) showed white blood count (WBC) count 18.4 × 109/L with 59% lymphocytes, hemoglobin 125 g/L, and platelet count 233 × 109/L. There was no lymphadenopathy or hepatosplenomegaly. Flow cytometry of blood lymphocytes revealed a clonal T-cell population expressing CD3, CD5, CD 45, and CD7, with coexpression of CD4 and CD8. These cells did not express CD34 or terminal deoxynucleotidyl transferase (tdt). T-cell receptor gene rearrangement study was positive. Studies for Epstein Barr virus were negative. Bone marrow was involved with disease. Initially, immunosuppressive therapy was reduced by 50%. Over the next 4 weeks, her fatigue worsened, she developed night sweats, and her lymphocyte count increased. Over the next eleven months, multiple therapies were tried without success (Figure 1). Approximately one year into her course she developed marked lymphocytosis and hepatosplenomegally.
Response of T-cell posttransplantation lymphoproliferative disorder to lenalidomide therapy. The response to therapy is depicted as absolute lymphocyte count. Patient had treatment failures to fludarabine; cyclophosphomide, prednisone, and vincristine (CVP), and cyclophosphomide, doxorubicin, prednisone, and vincristine (CHOP). Alemtuzumab resulted in a partial hematologic response before relapse. Lenalidomide resulted in a complete hematologic response. She ultimately was unable to tolerate lenalidomide therapy, resulting in disease progression and death.
Response of T-cell posttransplantation lymphoproliferative disorder to lenalidomide therapy. The response to therapy is depicted as absolute lymphocyte count. Patient had treatment failures to fludarabine; cyclophosphomide, prednisone, and vincristine (CVP), and cyclophosphomide, doxorubicin, prednisone, and vincristine (CHOP). Alemtuzumab resulted in a partial hematologic response before relapse. Lenalidomide resulted in a complete hematologic response. She ultimately was unable to tolerate lenalidomide therapy, resulting in disease progression and death.
After discussion with the patient, she was started on lenalidomide, a thalidomide derrivative at 25 mg/day. Lenalidomide is an immunomodulatory agent that augments T-cell response, increases secretion of tumor necrosis factor-α and interleukin-12, and suppresses angiogenesis.5 Thalidomide and lenalidomide have been shown to have activity against T-cell lymphomas.6,7 After 7 weeks of lenalidomide, our patient's lymphocyte count became normal (Figure 1). She reported subjective improvement and her hepatosplenomegally resolved. After 6 months, severe muscle cramps necessitated decrease of the lenalidomide dose to 10 mg every other day. Her cramps persisted, requiring discontinuation of treatment. This was followed by relapse of disease in 3 weeks (Figure 1). Resumption of lenalidomide at 10 mg twice a week again resulted in improvement of lymphocytosis. After 9 months, with patient unable to tolerate lenalidomide treatment, her disease progressed rapidly with heavy hepatic infiltration of leukemic cells. The patient declined further therapy and expired in 2 weeks.
Our patient achieved a complete hematologic response with lenalidomide therapy for 9 months, with normalization of her WBC count at optimal doses. We believe that this is the first report of a patient with T-cell PTLD obtaining response with lenalidomide therapy. This case shows a clear need for further investigation into the role of lenalidomide in T-cell PTLD.
Authorship
Conflict-of-interest disclosure: S.N. has been a consultant to Celgene Corporation. C.P. declares no competing financial interests.
Correspondence: Sucha Nand, Loyola University Medical Center, 2160 South First Avenue, Maywood, Illinois 60153; e-mail: snand@lumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal