Abstract
C-type lectin receptors (CLRs) fulfill multiple functions within the immune system by recognition of carbohydrate moieties on foreign or (altered) self-structures. CLRs on myeloid dendritic cells (DCs) have been well characterized as pattern-recognition receptors (PRRs) combining ligand internalization with complex signaling events. Much less is known about CLR expression and function in human plasmacytoid DCs (pDCs), the major type I interferon (IFN) producers. In this study, we demonstrate that, next to the CLR BDCA-2, human pDCs express DC immunoreceptor (DCIR), a CLR with putative immune-inhibitory function, but not dectin-1, mannose receptor, or DC-specific ICAM-3–grabbing nonintegrin. DCIR surface levels are reduced on pDC maturation after TLR9 triggering. Interestingly, DCIR triggering inhibits TLR9-induced IFN-α production while leaving up-regulation of costimulatory molecule expression unaffected. Furthermore, DCIR is readily internalized into pDCs after receptor triggering. We show that DCIR internalization is clathrin-dependent because it can be inhibited by hypertonic shock and dominant-negative dynamin. Importantly, antigens targeted to pDCs via DCIR are presented to T cells. These findings indicate that targeting DCIR on pDCs not only results in efficient antigen presentation but also affects TLR9-induced IFN-α production. Collectively, the data show that targeting of DCIR can modulate human pDC function and may be applied in disease preven-tion and treatment.
Introduction
C-type lectin receptors (CLRs) recognize defined carbohydrate moieties, the “C” indicating Ca2+ dependence of carbohydrate binding. Ligands may be of endogenous or exogenous nature.1 The prototypic CLR, dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN), has been shown to bind to high-mannose N-linked carbohydrates. DC-SIGN ligands are expressed by various pathogens, such as the fungus Candida albicans, HIV-1, bacteria, and protozoa.2 In addition, endogenous ligands such as intercellular adhesion molecule (ICAM)-2 and ICAM-3 have been identified.1 In antigen-presenting cells (APCs), CLRs and Toll-like receptors (TLRs) play an important role as pattern-recognition receptors (PRRs) by recognizing conserved pathogen-associated molecular patterns.3 Interestingly, because most CLRs are rapidly internalized after ligand-binding monoclonal antibodies (mAbs) directed against CLRs are currently exploited to target APCs.4-6
Plasmacytoid DCs (pDCs), also known as natural interferon-producing cells, are APCs playing an important role in the immune balance.7,8 Resting pDCs induce mostly regulatory responses, whereas activated pDCs secrete large amounts of interferon-α (IFN-α) and IFN-β as well as chemokines.7,9 To identify microbial intruders, they predominantly express TLR7 and TLR9, allowing them to respond to single-stranded RNA and DNA viruses.10 PDCs induce Th1 polarization11 and are crucial for initiating and sustaining antiviral immune responses by activating NK cell–mediated lysis of infected cells and by protecting uninfected cells from NK cytolytic activity. PDCs efficiently present viral antigens after infection and induce virus-specific primary and secondary CD4+ and CD8+ T-cell responses.12 Still little is known about antigen uptake by pDCs. Recently, a cooperative interaction between TLR9 and FcγRII on human pDCs was demonstrated in response to DNA-containing immune complexes isolated from patients with systemic lupus erythematosus. FcγR-mediated uptake of these immune complexes into endosomes containing TLR9 led to pDC activation.13 Furthermore, we have recently shown that pDCs can also take up exogenous antigens and present them to CD4+ T cells after FcγRII-mediated uptake.14
Increasing evidence suggests that pDCs express not only TLRs for pathogen-recognition and FcγRII for uptake of opsonized antigens, but also CLRs. So far, functional expression of the CLR BDCA-2 by human pDCs has been demonstrated. Antibody triggering induced BDCA-2 internalization and inhibited TLR9-mediated IFN-α production.15 Interestingly, BDCA-2 does not contain an intracellular signaling motif. Among the known human CLRs, DCIR and DC-associated c-type lectin 2 (DCAL-2) are the only ones containing intracellular immune receptor tyrosine-based inhibition motives (ITIMs). DCAL-2 expression is restricted to myeloid DCs,16 whereas DCIR expression has been demonstrated on various APCs, such as B cells, monocytes, and myeloid DCs.17 DCIR ligands have not yet been identified. Initial functional studies in B cells with chimeric molecules containing the DCIR cytoplasmic domain indicated that coligation of the B-cell receptor with the chimeric DCIR molecules led to a decrease in overall protein tyrosine phosphorylation and reduced Ca2+ flux.18
In the present study, we demonstrate that DCIR is expressed by human pDCs. Moreover, DCIR targeting inhibits TLR9-induced IFN-α production and results in antigen uptake and presentation by human pDCs.
Methods
Cells and reagents
All reagents were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands) unless indicated otherwise. PDCs were isolated from peripheral blood mononuclear cells by positive selection with anti-BDCA-4 antibody-coated magnetic beads (Miltenyi Biotec, Utrecht, The Netherlands) and cultured in X-Vivo15 medium (Cambrex, Verviers, Belgium)/2% human serum and 10 ng/mL interleukin-3 (IL-3). HEK293 cells were cultured in complete Dulbecco modified Eagle medium (Invitrogen, Breda, The Netherlands)/10% fetal calf serum and CHO cells in Ham's F12 medium (Invitrogen)/10% fetal calf serum. Transfections were done with lipofectamine (Invitrogen). CHO cells stably expressing DCIR were selected with geneticin (Clontech, Mountain View, CA).
Antibodies
Polyclonal goat (R&D Systems, Minneapolis, MN) antihuman DCIR antibodies were used for fluorescence-activated cell sorting (FACS) analysis and confocal laser scanning microscopy (CLSM). Monoclonal murine antihuman DCIR antibodies (clone 216110; R&D Systems) were used to cross-link DCIR in functional studies. Human pDCs were labeled with murine antihuman BDCA-2 antibodies (clone AC144; Miltenyi Biotec). FACS analyses were performed with a FACScalibur (Clontech). FACS data were analyzed with WinMDI (http://facs.scripps.edu/software.html) and CellQuest (Clontech). MHC class I and II and CD40, CD80 and CD86 molecules were stained with mouse monoclonal antibodies (clone W6/32; American Type Culture Collection, Manassas, VA; clone Q5/13/clone SPV-L3, clone MAB89; Beckman Coulter, Mijdrecht, The Netherlands; and clone L307.4 and clone 2331 (FUN-1); Clontech, respectively). Human DC-SIGN was detected with murine anti-DC-SIGN (clone AZND1; Beckman Coulter), human dectin-1 with goat polyclonal (R&D Systems) and human MR with murine monoclonal antibodies (clone 19.2; Clontech). Rabbit polyclonal anti-keyhole limpet hemocyanin (KLH) antibody was kindly provided by Frank van Leeuwen, Children's Hospital, University Medical Center, Nijmegen, The Netherlands. Secondary antibodies for FACS analysis were PE- (Clontech) or Alexa647-labeled (Invitrogen). For confocal imaging, donkey antigoat Alexa647- and isotype-specific goat antimouse Alexa488- and Alexa647-labeled secondary antibodies were used (Invitrogen).
cDNA constructs
cDNA was generated from mRNA of human immature monocyte-derived DCs. The following DCIR primers were used in PCR reactions (forward primer: 5′-ATG ACT TCG GAA ATC ACT TAT G-3′, reverse primer: 5′-CTT GGG GAA TCC GGT ATT AC-3′). The DCIR coding sequence was cloned into a pEGFP-N1 vector in which EGFP had been replaced by a hemagglutinin (HA)-tag or into pEGFP-C1 (Clontech). Constructs were checked for correct reading frame and mutations at the department of Anthropogenetics (NCMLS, Nijmegen, The Netherlands). The human BDCA-2 construct was a kind gift of J. Schmitz (Miltenyi Biotec). The human HA-tagged clathrin wild-type and dominant-negative constructs were generously provided by Onno Kranenburg (Department of Surgery, University Medical Center Utrecht, Utrecht, The Netherlands).
Western blot
Cells were lysed in lysis buffer containing 150 mM NaCl, 10 mM Tris HCl, pH 7.5, 2 mM MgCl2, 1% Triton X-100, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride; 5% β-mercaptoethanol was added. Samples were separated by polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes. Membranes were stained with goat anti-DCIR antibodies. Antibody signals were detected with horse radish peroxidase-coupled secondary antibodies using an enhanced luminescence detection kit (Pierce Chemical, Rockford, IL).
DCIR detection by confocal laser scanning microscopy
CHO-DCIR-EGFP cells were allowed to adhere to fibronectin-coated coverslips, fixed in 1% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100. Cells were incubated with murine monoclonal anti-DCIR antibodies for 30 minutes at 4°C. Unbound antibodies were washed away. Cells were stained with goat antimouse IgG1-Alexa488. Isotype control antibodies did not show any staining. Cells were mounted in mowiol and analyzed by CLSM (MRC1024; Bio-Rad, Hercules, CA).
DCIR cross-linking assays
Flat-bottom 96-well plates were coated with murine anti-DCIR or matched isotype control antibodies (mIgG1) at a concentration of 10 μg/mL in PBS for 2 hours at 37°C. Unbound antibody was discarded. The plates were washed once with PBS before adding the pDCs. PDCs were incubated with TLR ligands or control medium for 6 to 24 hours. Cytokine secretion and expression of pDC activation markers were analyzed.
DCIR internalization assays
Cells were incubated with goat polyclonal anti-DCIR, murine monoclonal anti–BDCA-2 or anti-MHC class I antibodies (10 μg/mL) in medium for 30 minutes on ice. Unbound antibodies were washed away, and cells were incubated at 37°C or 4°C for various time points. The amount of Ab complexes remaining on the cell surface was then determined by FACS analysis after staining cells with antimouse IgG-PE or antigoat-IgG-Alexa647 secondary antibodies.
To detect internalized DCIR or BDCA-2 protein, cells were first incubated for 30 minutes on ice with polyclonal goat anti-DCIR or monoclonal anti-BDCA-2 antibodies followed by incubation with donkey antigoat-IgG-Alexa647 or goat antimouse-IgG1-Alexa647 for 30 minutes on ice. After incubation at 37°C and 4°C for various time points, cells were fixed, mounted on poly-L-lysine-coated coverslips, and counterstained with mouse anti-major histocompatibility complex (MHC) class I (10 μg/mL) antibodies followed by goat-antimouse IgG2a-Alexa488 for CLSM. For detection of internalized receptors by FACS, surface antibodies were stripped off by shifting pH to 2.5. To inhibit clathrin-mediated endocytosis, cells were incubated in hypertonic media. Incubation in media containing 450 mM sucrose results in disruption of clathrin-coated pits and induces formation of nonfunctional so-called clathrin microcages. This process is fully reversible once cells are returned into isotonic media.19,20
Antigen targeting
PBLs and pDCs were isolated from melanoma patients participating in ongoing DC vaccination trials.21 The study was approved by the Institutional Review Board of Radboud University Nijmegan Medical Center, Commissie Mensgebonden Onderzoek, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Antigen-pulsed monocyte-derived DCs (gp100 peptides, tyrosinase peptide, and KLH) were administered intravenously and intradermally. PDCs were isolated from apheresis products and incubated at 4°C with anti–DCIR-mAb- and anti-DC-SIGN-mAb-KLH (both murine IgG1) conjugates generated as described previously.5 Binding of both conjugates to DCIR was examined with a CHO cell line stably expressing DCIR and untransfected CHO cells. Cells were labeled for 30 minutes on ice with the conjugates, washed, and counterstained with rabbit polyclonal anti-KLH and goat antirabbit-IgG-Alexa647 antibodies. To block anti–DCIR-mAb-KLH binding, pre-incubations with anti-DCIR or isotype control antibodies were performed. As control, pDCs were incubated with KLH or with KLH and patient-derived postvaccination serum containing anti-KLH antibodies. PDCs were washed and cocultured with KLH-responsive PBLs at 37°C. Pretreatment with anti-DCIR antibody was performed for 30 minutes at 4°C followed by incubation with anti–DCIR-mAb-KLH conjugates. All pDCs were cultured in IL-3. CpG-C (5 μg/mL) was added to pDC samples. After 4 days of coculture, a titrated thymidine incorporation assay was performed. Titrated thymidine (1 μCi/well; MP Biomedicals, Eindhoven, The Netherlands) was added to the cell cultures and incorporation was measured after 16 hours.
Cytokine production
pDCs (5 × 104) were stimulated with CpG-C M-352 (5 μg/mL). Supernatants were collected after 6 to 24 hours, and IFN-α production was analyzed with murine monoclonal capture and HRP-conjugated anti-IFN-α antibodies (Bender, Vienna, Austria) using standard ELISA procedures. IL-6, IL-10, IL-12, and tumor necrosis factor-α (TNF-α) production were measured using the Luminex technology, a beads-based cytokine detection system, according to the manufacturer's instructions (Bio-Rad/Luminex, Oosterhout, The Netherlands).
Statistical analyses
Statistical differences were determined using independent-samples t test procedure. Significance was accepted at P levels of at least .05.
Results
Human pDCs express the CLR DCIR
To study CLR expression, pDCs were isolated by positive selection using anti–BDCA-4-antibody–coated magnetic beads. Isolated cell populations were highly pure as 95% to 99% of the cells expressed the pDC marker BDCA-2 (Figure 1). Freshly isolated pDCs were then stained with anti–DCIR, anti–dectin-1, anti–DC-SIGN, or anti-Mannose receptor (MR) antibodies and analyzed by flow cytometry. PDCs clearly express DCIR, whereas dectin-1, DC-SIGN, and MR are not detectable (Figure 1). Because the extracellular domains of BDCA-2 and DCIR are 42% identical,15 cross-reactivity of the anti-DCIR antibodies with BDCA-2 had to be excluded. To this end, HEK293 cells were transiently transfected with BDCA-2 or DCIR cDNA constructs and labeled with the antibodies. Neither the goat nor murine anti-DCIR antibodies recognized BDCA-2. Conversely, anti–BDCA-2 antibody did not bind to DCIR. The latter finding is in line with literature reporting no binding of anti–BDCA-2 antibodies to DCIR-expressing cells, such as monocytes22 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Human pDCs thus express the CLR DCIR, a receptor containing an immune inhibitory motif.
C-type lectin DCIR is expressed by human pDCs. PDCs were isolated with anti–BDCA-4 antibody-coated magnetic beads and labeled with the indicated antibodies (shaded areas) or the matching isotype controls (dotted lines). Samples were analyzed by flow cytometry and gated on the pDC population (according to forward/sideward scatter). DCIR and BDCA-2 are clearly expressed, whereas expression of the CLRs DC-SIGN, MR, and dectin-1 cannot be detected. FACS data are representative of 4 independent experiments.
C-type lectin DCIR is expressed by human pDCs. PDCs were isolated with anti–BDCA-4 antibody-coated magnetic beads and labeled with the indicated antibodies (shaded areas) or the matching isotype controls (dotted lines). Samples were analyzed by flow cytometry and gated on the pDC population (according to forward/sideward scatter). DCIR and BDCA-2 are clearly expressed, whereas expression of the CLRs DC-SIGN, MR, and dectin-1 cannot be detected. FACS data are representative of 4 independent experiments.
TLR9-induced pDC maturation reduces DCIR expression
To study the effects of pDC maturation on CLR expression, freshly isolated pDCs were cultured in medium containing IL-3 plus the TLR9 ligand CpG-C. PDC activation using these conditions led to a significant reduction of DCIR and BDCA-2 surface levels to 31% plus or minus 14% for DCIR (P ≤ .001) and to 14% plus or minus 10% (P ≤ .001) for BDCA-2 (Figure 2A top and bottom panels). Dectin-1 remained undetectable after stimulation with CpG-C. CpG-C induced robust pDC maturation as indicated by increased CD80/CD86 expression and IFN-α secretion (data not shown). Next to TLR9, human pDCs are known to express only TLR7.8,23 We therefore also included the TLR7/8 ligand R848 and the TLR7 ligand loxoribine to analyze the effects of TLR7 triggering on DCIR expression in pDCs. TLR7 stimulation by both compounds reduced DCIR expression on pDCs to a similar extent as TLR9 triggering with CpG-C (data not shown). We note that incubation in medium containing IL-3 already leads to a decrease in DCIR and BDCA-2 surface levels (data not shown), in line with the finding that IL-3 induces transition from pre-pDCs to pDCs.24 Addition of the TLR9 ligand CpG-C, however, further reduces DCIR and BDCA-2 expression.
DCIR triggering does not affect TLR9-mediated costimulatory molecule expression but inhibits IFN-α production. (A) TLR9 triggering induces BDCA-2 and DCIR down-regulation. Expression of BDCA-2, DCIR and dectin-1 was analyzed directly after cell isolation (left) and after 24 hours of culture with IL-3 and CpG-C (right). Dectin-1 is not expressed under the conditions tested. The numbers indicate Δ MFI (MFI of specific antibody minus MFI of matched isotype control). Data represent 3 independent experiments (top panel). BDCA-2 and DCIR down-regulation in response to TLR9 triggering with CpG-C is statistically significant with a reduction to 14% ± 10% (*P ≤ .001, data represent the mean ± SEM of 3 experiments) for BDCA-2 and to 31% ± 14% for DCIR (*P ≤ .001, data represent the mean ± SEM of 6 experiments; bottom panel). (B) TLR9-induced pDC maturation is not influenced by DCIR triggering. Freshly isolated pDCs were stimulated with 5 μg/mL CpG-C or medium on plates coated with murine anti-DCIR or matching isotype control antibodies. Cells were harvested after 24 hours and stained with anti-CD80, anti-CD86, and anti-CD40 (shaded areas) or isotype control antibodies (dotted lines). The maturation markers CD40 and CD80 were not expressed on resting pDCs, whereas there was some CD86 expression detectable. Expression of all costimulatory molecules was quickly up-regulated in CpG-C stimulated cells irrespective of DCIR triggering. Similar results were obtained for MHC class II (data not shown). The data represent 3 independent experiments. (C) DCIR triggering inhibits TLR9-mediated IFN-α production by human pDCs. PDCs were incubated on plates coated with murine anti-DCIR or isotype control antibodies. Cells were stimulated with CpG-C (5 μg/mL) or left unstimulated (control), and IFN-α production was analyzed after 6 and 8 hours (left, one representative donor). DCIR triggering inhibited IFN-α production by 40% ± 3% compared with isotype control samples (P ≤ .001, right, data represent the mean ± SEM of 3 independent experiments; incubation time 8-24 hours). (D) DCIR triggering inhibits TNF-α but not IL-6 production. Cells were treated as in panel C) and TNF-α and IL-6 secretion were analyzed. DCIR triggering inhibited TNF-α production by 28% ± 5% (P ≤ .002), whereas it did not affect IL-6 production (data represent the mean ± SEM of 3 independent experiments; incubation time 6-24 hours). There was no production of IL-10 or IL-12 detectable (data not shown).
DCIR triggering does not affect TLR9-mediated costimulatory molecule expression but inhibits IFN-α production. (A) TLR9 triggering induces BDCA-2 and DCIR down-regulation. Expression of BDCA-2, DCIR and dectin-1 was analyzed directly after cell isolation (left) and after 24 hours of culture with IL-3 and CpG-C (right). Dectin-1 is not expressed under the conditions tested. The numbers indicate Δ MFI (MFI of specific antibody minus MFI of matched isotype control). Data represent 3 independent experiments (top panel). BDCA-2 and DCIR down-regulation in response to TLR9 triggering with CpG-C is statistically significant with a reduction to 14% ± 10% (*P ≤ .001, data represent the mean ± SEM of 3 experiments) for BDCA-2 and to 31% ± 14% for DCIR (*P ≤ .001, data represent the mean ± SEM of 6 experiments; bottom panel). (B) TLR9-induced pDC maturation is not influenced by DCIR triggering. Freshly isolated pDCs were stimulated with 5 μg/mL CpG-C or medium on plates coated with murine anti-DCIR or matching isotype control antibodies. Cells were harvested after 24 hours and stained with anti-CD80, anti-CD86, and anti-CD40 (shaded areas) or isotype control antibodies (dotted lines). The maturation markers CD40 and CD80 were not expressed on resting pDCs, whereas there was some CD86 expression detectable. Expression of all costimulatory molecules was quickly up-regulated in CpG-C stimulated cells irrespective of DCIR triggering. Similar results were obtained for MHC class II (data not shown). The data represent 3 independent experiments. (C) DCIR triggering inhibits TLR9-mediated IFN-α production by human pDCs. PDCs were incubated on plates coated with murine anti-DCIR or isotype control antibodies. Cells were stimulated with CpG-C (5 μg/mL) or left unstimulated (control), and IFN-α production was analyzed after 6 and 8 hours (left, one representative donor). DCIR triggering inhibited IFN-α production by 40% ± 3% compared with isotype control samples (P ≤ .001, right, data represent the mean ± SEM of 3 independent experiments; incubation time 8-24 hours). (D) DCIR triggering inhibits TNF-α but not IL-6 production. Cells were treated as in panel C) and TNF-α and IL-6 secretion were analyzed. DCIR triggering inhibited TNF-α production by 28% ± 5% (P ≤ .002), whereas it did not affect IL-6 production (data represent the mean ± SEM of 3 independent experiments; incubation time 6-24 hours). There was no production of IL-10 or IL-12 detectable (data not shown).
DCIR triggering does not affect TLR9-induced costimulatory molecule expression by human pDCs
DCIR contains an intracellular ITIM motif suggested to play a role in immune inhibition. We therefore hypothesized that receptor triggering may inhibit immune activating processes. In response to activating stimuli, such as TLR9 ligands, pDCs express the costimulatory molecules CD40,25 CD80, and CD86, which play a central role in T-cell activation.26 Freshly isolated pDCs were cultured on plates coated with murine anti-DCIR or matching isotype control antibodies. Addition of CpG-C induced robust CD40, CD80, and CD86 up-regulation after 24 hours compared with medium controls. However, DCIR triggering did not influence expression of the costimulatory molecules (Figure 2B). The same was true for MHC class II (data not shown). Inhibition of TLR9-mediated CD40, CD80, or CD86 up-regulation is therefore not a mechanism of potential DCIR-mediated immune suppression.
DCIR triggering inhibits TLR9-mediated IFN-α production by human pDCs
Peptides comprising the phosphorylated cytoplasmic ITIM domain of DCIR have been shown to interact with the tyrosine phosphatase SHP-1,27 which inhibits cytokine signaling.28 PDCs produce large amounts of IFN-α during the first 12 hours of activation with TLR ligands.24 We therefore analyzed whether DCIR cross-linking would influence TLR9-mediated IFN-α production. PDCs were cultured on plates coated with anti-DCIR or matching isotype control antibodies and stimulated with CpG-C. DCIR-triggering clearly inhibited TLR9-induced IFN-α production by 40% (Figure 2C right) relative to isotype control. Inhibition could be detected after 6 to 8 hours in the time course experiment depicted in Figure 2C (left). Furthermore, DCIR-triggering also reduced TLR9-mediated TNF-α production by 28%, whereas IL-6 remained unaffected (Figure 2D). In line with recent reports in the literature, we did not detect any IL-10 or IL-12 production by human pDCs after TLR9-triggering.29,30 As described by Dzionek et al,15 triggering with anti–BDCA-2 antibodies performed in parallel experiments resulted in 80% to 90% reduced IFN-α production in response to CpG-C (data not shown). Because we had observed reduced DCIR expression on pDCs after TLR7 triggering, we also analyzed the effects of DCIR cross-linking on TLR7-mediated IFN-α production. We found that TLR7 triggering induced IFN-α production by pDCs, albeit much less and less consistent than TLR9 triggering (up to 70 times more IFN-α with CpG-C than with R848). However, we did not observe significant inhibition of TLR7-mediated IFN-α production by DCIR cross-linking (data not shown). In summary, DCIR-triggering inhibited TLR9-mediated IFN-α secretion by 40% plus or minus 3% (P ≤ .001, n = 3; Figure 2C right).
Cell surface DCIR is internalized into pDCs after receptor triggering
A putative mechanism for DCIR to inhibit TLR9-mediated IFN-α production could be modulation of TLR9 signaling events. This would probably require DCIR endocytosis and trafficking into endosomal compartments containing TLR9. To study this hypothesis, DCIR internalization experiments were performed. PDCs were incubated with anti-DCIR antibodies. Incubation at 37°C clearly reduced the cell surface level of DCIR by 50% compared with control cells incubated at 4°C (Figure 3A left). Under the same conditions, surface BDCA-2 expression readily decreased by 37%, whereas MHC class I showed only minor internalization. To visualize DCIR localization after antibody triggering, pDCs were analyzed by confocal laser scanning microscopy (CLSM). At 4°C, DCIR clearly colocalizes with MHC class I at the cell surface (Figure 3A right). Once the incubation temperature is elevated to 37°C, DCIR is internalized and can be detected in intracellular vesicular structures. In line with literature,15 BDCA-2 was also internalized (Figure 3A right).
Cell surface DCIR is internalized into pDCs after receptor triggering. (A) DCIR internalization is induced by receptor triggering with anti-DCIR antibodies. PDCs were labeled with anti-DCIR, anti-BDCA-2, or anti-MHC class I antibodies followed by secondary antibodies on ice. Incubation at 37°C induces receptor internalization leading to 50% reduced DCIR (MFI 9 vs 18) and 37% reduced BDCA-2 (MFI 89 vs 141) surface expression (thick line) compared with cells incubated at 4°C (shaded area, left). Under the same conditions, MHC class I expression is reduced by only 17% (MFI 710 vs 857). Matching isotype controls did not show any binding (dotted lines). FACS data are representative of 3 independent experiments (left). PDCs were labeled with anti-DCIR or anti–BDCA-2 antibodies, washed, and incubated on ice with fluorochrome-conjugated secondary antibodies (blue). To visualize the cell surface, pDCs were counterstained with murine anti-MHC class I antibodies and isotype-specific fluorochrome-conjugated secondary antibodies (green). Cells were analyzed by CLSM (right). DCIR can be detected at the cell surface after antibody labeling at 4°C and is clearly internalized after elevating the incubation temperature to 37°C. BDCA-2 endocytosis after receptor triggering is detected as described in the literature.15 (B) Incubation of pDCs in 450 mM sucrose inhibits DCIR endocytosis at 37°C, which is restored after washing out sucrose and incubating the cells in isotonic medium (sucrose washout). In each histogram, total surface protein (gray shaded area) is compared with internalized antibody (thick line) after stripping off surface-bound antibodies, as described in “Methods.” A matched isotype control did not bind to the cells (dotted lines). Data shown are representative of 3 independent experiments.
Cell surface DCIR is internalized into pDCs after receptor triggering. (A) DCIR internalization is induced by receptor triggering with anti-DCIR antibodies. PDCs were labeled with anti-DCIR, anti-BDCA-2, or anti-MHC class I antibodies followed by secondary antibodies on ice. Incubation at 37°C induces receptor internalization leading to 50% reduced DCIR (MFI 9 vs 18) and 37% reduced BDCA-2 (MFI 89 vs 141) surface expression (thick line) compared with cells incubated at 4°C (shaded area, left). Under the same conditions, MHC class I expression is reduced by only 17% (MFI 710 vs 857). Matching isotype controls did not show any binding (dotted lines). FACS data are representative of 3 independent experiments (left). PDCs were labeled with anti-DCIR or anti–BDCA-2 antibodies, washed, and incubated on ice with fluorochrome-conjugated secondary antibodies (blue). To visualize the cell surface, pDCs were counterstained with murine anti-MHC class I antibodies and isotype-specific fluorochrome-conjugated secondary antibodies (green). Cells were analyzed by CLSM (right). DCIR can be detected at the cell surface after antibody labeling at 4°C and is clearly internalized after elevating the incubation temperature to 37°C. BDCA-2 endocytosis after receptor triggering is detected as described in the literature.15 (B) Incubation of pDCs in 450 mM sucrose inhibits DCIR endocytosis at 37°C, which is restored after washing out sucrose and incubating the cells in isotonic medium (sucrose washout). In each histogram, total surface protein (gray shaded area) is compared with internalized antibody (thick line) after stripping off surface-bound antibodies, as described in “Methods.” A matched isotype control did not bind to the cells (dotted lines). Data shown are representative of 3 independent experiments.
DCIR internalization is clathrin-dependent
Next, the mechanism underlying DCIR internalization was investigated in more detail. Receptor-mediated endocytosis is often clathrin-mediated.31 Clathrin-dependent endocytosis can be inhibited by incubation in hypertonic media. PDC incubation with anti-DCIR and fluorochrome-conjugated secondary antibodies at 37°C induced DCIR internalization. Disruption of clathrin-coated pits in hyperosmolar medium resulted in total inhibition of DCIR internalization in pDCs, which was fully restored after sucrose washout (Figure 3B).
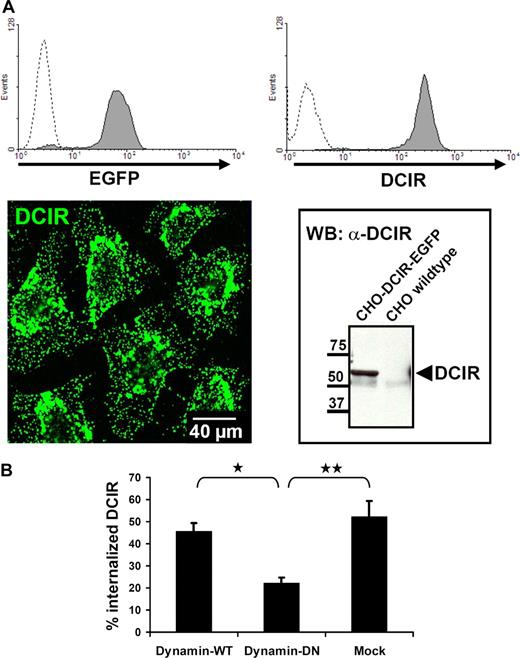
The low frequency of pDCs in peripheral blood hampers their biochemical analysis. Therefore, to further substantiate clathrin-dependence of DCIR endocytosis, a CHO cell line stably expressing human DCIR-EGFP was generated (Figure 4A). Vesicle fissure in clathrin-mediated endocytosis is known to be dynamin-dependent. CHO-DCIR-EGFP cells were cotransfected with dynamin–wild-type (WT) or -dominant negative (DN) constructs. Cotransfection of DN-dynamin resulted in 50% inhibition of DCIR internalization (Figures 4B,S2). Together, these data reveal clathrin dependence of DCIR internalization.
DCIR internalization is clathrin-dependent. (A) For further analysis of DCIR endocytosis, a CHO cell line stably expressing human DCIR-EGFP was generated. DCIR expression was detected by FACS (top panel), either via EGFP detection (left, dotted line represents untransfected cells) or after labeling with anti-DCIR antibodies (right, dotted line represents isotype control). DCIR could also be detected by CLSM (bottom panel, left) and Western blot analysis (right). (B) DCIR internalization is inhibited by DN-dynamin. Cotransfection of the DCIR expressing CHO cell line with DN-dynamin constructs reduces DCIR endocytosis by 50% compared with cells cotransfected with WT-dynamin (*P ≤ .009) or to mock-transfected cells (**P ≤ .05). The data shown represent the mean (± SEM) of 3 independent experiments.
DCIR internalization is clathrin-dependent. (A) For further analysis of DCIR endocytosis, a CHO cell line stably expressing human DCIR-EGFP was generated. DCIR expression was detected by FACS (top panel), either via EGFP detection (left, dotted line represents untransfected cells) or after labeling with anti-DCIR antibodies (right, dotted line represents isotype control). DCIR could also be detected by CLSM (bottom panel, left) and Western blot analysis (right). (B) DCIR internalization is inhibited by DN-dynamin. Cotransfection of the DCIR expressing CHO cell line with DN-dynamin constructs reduces DCIR endocytosis by 50% compared with cells cotransfected with WT-dynamin (*P ≤ .009) or to mock-transfected cells (**P ≤ .05). The data shown represent the mean (± SEM) of 3 independent experiments.
DCIR-mediated uptake leads to antigen presentation
Because pDCs rapidly internalized DCIR on mAb binding, we evaluated the induction of T-cell responses to KLH by targeting DCIR on human pDCs. Hereto, we generated anti–DCIR-mAb-KLH (anti–DCIR-KLH) and anti–DC-SIGN-mAb-KLH (anti–DC-SIGN-KLH) conjugates. Whereas anti–DCIR-KLH bound DCIR-expressing CHO cells, anti–DC-SIGN-KLH did not (Figure 5A left). Anti–DC-SIGN-KLH was functional, as it did bind to monocyte-derived DCs (data not shown). The specificity of these findings was confirmed by blocking with anti-DCIR antibodies but not by preincubation with isotype control (Figure 5A, right). Next, pDCs and PBLs were isolated from apheresis products of melanoma patients participating in ongoing clinical DC vaccination trials that include KLH as a helper antigen. Patient-derived isolated pDCs were loaded on ice with protein conjugates consisting of KLH and DCIR-mAb, KLH and DC-SIGN-mAb, or KLH alone. As a positive control, KLH was targeted to FcγRII via anti-KLH IgG antibodies.14 PDCs were then incubated with IL-3 and matured with CpG-C. Fresh patient-derived PBLs were added to the pDCs, and proliferation was measured after 4 days of coculture without adding any exogenous cytokines. Strikingly, KLH targeting via DCIR induced a strong proliferative recall response and was as effective as targeting via FcγRII. Moreover, pDC preincubation with anti-DCIR antibodies blocked the observed targeting effect to background levels demonstrating the specificity of the response. Because pDCs do not take up soluble exogenous antigens in a receptor-independent fashion,32 anti–DC-SIGN-mAb-KLH conjugates or KLH alone did not induce proliferation above background levels (Figure 5B). DCIR is thus not only internalized after cross-linking, but it also delivers its antigenic cargo into the antigen presentation pathway of pDC resulting in T-cell proliferation.
Human pDCs present KLH to PBLs after DCIR-mediated uptake. (A) Anti–DCIR-KLH binds specifically to DCIR-expressing CHO cells. The CHO cell line stably expressing human DCIR was incubated on ice with anti–DCIR-KLH, anti–DC-SIGN-KLH, rabbit polyclonal anti-KLH, and fluorochrome-conjugated secondary antibodies. FACS analysis showed that anti–DCIR-KLH binds well to the CHO-DCIR cell line, whereas binding of the anti–DC-SIGN conjugate cannot be detected (left). Binding of anti–DCIR-KLH (gray shaded area) can be inhibited by preincubation with anti-DCIR antibodies (thick line). Preincubation with a matching isotype control (dashed line) does not block anti–DCIR-KLH binding (right). Anti–DCIR-KLH does not bind to untransfected CHO cells (data not shown). (B) PDCs were freshly isolated and loaded with anti–DC-SIGN-KLH and anti–DCIR-KLH conjugates, KLH alone, or KLH together with patient-derived serum containing anti-KLH antibodies (positive control). To block DCIR-mediated antigen uptake, pDCs were also preincubated with anti-DCIR mAb before antigen targeting. PDCs were cultured with IL-3 and matured with 5 μg/mL of CpG-C. Fresh PBLs were added and thymidine incorporation was measured after 4 days of coculture. DCIR targeting induces PBL proliferation clearly above background of pDCs loaded with KLH alone or anti–DC-SIGN-KLH (*P ≤ .012 and **P ≤ .019, respectively). Proliferation can be blocked by preincubation with anti-DCIR antibody (***P ≤ .051). Similar data were obtained with pDCs incubated without CpG-C (data represent mean ± SEM of experiments performed in triplicate). Similar data were obtained from 2 independent patients.
Human pDCs present KLH to PBLs after DCIR-mediated uptake. (A) Anti–DCIR-KLH binds specifically to DCIR-expressing CHO cells. The CHO cell line stably expressing human DCIR was incubated on ice with anti–DCIR-KLH, anti–DC-SIGN-KLH, rabbit polyclonal anti-KLH, and fluorochrome-conjugated secondary antibodies. FACS analysis showed that anti–DCIR-KLH binds well to the CHO-DCIR cell line, whereas binding of the anti–DC-SIGN conjugate cannot be detected (left). Binding of anti–DCIR-KLH (gray shaded area) can be inhibited by preincubation with anti-DCIR antibodies (thick line). Preincubation with a matching isotype control (dashed line) does not block anti–DCIR-KLH binding (right). Anti–DCIR-KLH does not bind to untransfected CHO cells (data not shown). (B) PDCs were freshly isolated and loaded with anti–DC-SIGN-KLH and anti–DCIR-KLH conjugates, KLH alone, or KLH together with patient-derived serum containing anti-KLH antibodies (positive control). To block DCIR-mediated antigen uptake, pDCs were also preincubated with anti-DCIR mAb before antigen targeting. PDCs were cultured with IL-3 and matured with 5 μg/mL of CpG-C. Fresh PBLs were added and thymidine incorporation was measured after 4 days of coculture. DCIR targeting induces PBL proliferation clearly above background of pDCs loaded with KLH alone or anti–DC-SIGN-KLH (*P ≤ .012 and **P ≤ .019, respectively). Proliferation can be blocked by preincubation with anti-DCIR antibody (***P ≤ .051). Similar data were obtained with pDCs incubated without CpG-C (data represent mean ± SEM of experiments performed in triplicate). Similar data were obtained from 2 independent patients.
Discussion
PDCs are the major type I IFN producers and play a crucial role in the antiviral immune defense.8,33 To detect invading pathogens, they express receptors, such as TLR7 and 9 and FcγR II. Still little is known about the role of CLRs in human pDC biology. In this study, we demonstrate not only that human pDCs express the CLR DCIR, but also that DCIR triggering inhibits TLR9-induced IFN-α production. Our data show that DCIR is internalized into pDCs on receptor triggering and that internalization is clathrin-dependent. Antigen uptake via DCIR leads to antigen presentation and subsequent T-cell activation. DCIR is thus a new endocytic human pDC receptor that cross-talks with TLR9.
The data presented show that DCIR is expressed by human pDCs, whereas expression of the CLRs dectin-1, DC-SIGN, and MR could not be detected. DCIR triggering does not affect expression of the pDC maturation markers CD40, CD80, and CD86. However, DCIR triggering down-regulates TLR9-mediated IFN-α production by pDCs. TLR9 signals via MyD88, IRAK1, IRAK4, and TRAF6 resulting in IRF7 and downstream NFκ-B and AP-1 activation.34 The molecular mechanism used by the ITIM-containing DCIR to inhibit TLR9-mediated IFN-α production by pDCs has not yet been identified but probably involves receptor internalization. We have observed that after simultaneous incubation with fluorescent CpG-C and anti-DCIR antibodies both colocalize intracellularly in human pDCs (data not shown). A possible explanation for the observed inhibitory effects could thus be recruitment of inhibitory phosphatases such as SHP-1 via the ITIM of DCIR bringing them in close vicinity to CpG-C–activated TLR9. Similarly, the mechanism used by BDCA-2 to inhibit TLR9 signaling has not been elucidated.15 However, BDCA-2 does not contain an intracellular signaling motif. Its mode of action may therefore be different from the one used by DCIR. DCIR is an immune inhibitory receptor; and together with other receptors, it will play a role in maintaining immune balance. The situation is quite similar to the maintenance of immune balance by Fc receptors, where the relative amounts of inhibitory and activating receptors determine whether the antibody-mediated antigen uptake results in tolerance or immunity.35 Interestingly, the finding that TLR9 triggering on pDCs in turn down-regulates DCIR cell surface levels implies a reciprocal relationship between TLR9-mediated immune activation and the inhibitory effects of DCIR. The reduction of DCIR surface levels in response to TLR9 stimulation is at least in part mediated via IFN-α because it induced DCIR down-regulation by 78% compared with a down-regulation by 74% induced by CpG-C. Addition of TNF-α, on the other hand, did not lead to significant reduction of DCIR expression (data not shown).
PDCs are known to express only TLR7 next to TLR9.8,23 In initial experiments, we therefore also included the TLR7/8 ligand R848 and the TLR7 ligand loxoribine. TLR7 stimulation by both compounds reduced DCIR expression on pDCs to a similar extent as TLR9 triggering with CpG-C (data not shown). In line with these data, we found that TLR7 triggering also induced IFN-α production by pDCs, albeit much less than TLR9 triggering. However, we did not observe significant inhibition of IFN-α production after TLR7 activation.
Cross-talk between TLRs and CLRs on myeloid DCs has been demonstrated for the immune receptor tyrosine-based activation motif (ITAM)–containing dectin-1 and TLR2 and results in greatly increased TNF-α production.36,37 Dectin-1 is an APC β-glucan receptor that is functionally regulated by tetra-spanin CD37.38 Furthermore, the CLR DC-SIGN has been shown to inhibit TLR activation of myeloid DCs by Mycobacterium tuberculosis after binding to its cell wall component ManLAM.39 Cross-talk between CLRs and TLRs thus represents a more general mechanism to fine-tune the function of myeloid DCs and now also pDCs.
DCIR function in pDCs goes further than inhibiting TLR9 signaling. The data presented show that DCIR functions as an endocytic receptor on human pDCs and that it is connected to the clathrin-mediated receptor uptake machinery, which further supports the notion that DCIR delivers its cargo to the endosomal/lysosomal compartments.40 CLRs have been shown to mediate pathogen uptake.2 Recently, antibody targeting of DEC-2056 and DC-SIGN,5 2 CLRs expressed by human myeloid DCs, has been used successfully for antigen delivery to human DCs. Uptake of a DEC-205/HIV p24 targeting vehicle by human DCs stimulated proliferation and IFN-γ production by CD8+ T cells isolated from the blood of HIV-infected donors. DC-SIGN–mediated targeting of KLH to human monocyte-derived DCs induced proliferation of T cells recognizing KLH epitopes in the context of MHC class I and II. Antibody-mediated antigen targeting to DCs via DC-SIGN effectively induced antigen-specific naive as well as recall T-cell responses. Targeted antigen delivery to DCs via CLRs is thus a promising strategy to increase efficiency of vaccines against infectious and malignant diseases.41
Our data now show that endocytosis of the exogenous antigen KLH via DCIR leads to efficient antigen presentation evoking a genuine recall response from fresh T cells isolated directly from peripheral blood of KLH-vaccinated melanoma patients after a coculture time of only 4 days. The absence of T-cell proliferation above background levels when pDC were targeted via the myeloid DC-specific lectin DC-SIGN emphasizes the specificity of targeting via DCIR. The mechanisms of antigen presentation by human pDCs are still much less understood. Because pDCs do not take up soluble antigens in a receptor-independent fashion,7,32 receptor-mediated uptake is crucial for antigen presentation by pDCs. We have recently shown that FcgRII-mediated antigen uptake leads to efficient antigen presentation by human pDCs.14 Furthermore, targeting of the pDC via a murine monoclonal anti–BDCA-2 antibody induced proliferation of a murine IgG-responsive T-cell line.15 Our data now show that DCIR-mediated antigen uptake by pDCs induces a memory T-cell response comparable with that achieved via FcγRII-mediated antigen internalization. Importantly, as DCIR is expressed by human pDCs as well as myeloid DCs and B cells, it could in principle be used to target multiple APC types simultaneously.
A previous human study by Gilliet et al as well as our own data have shown that the proliferative anti-KLH response in the patient-derived PBMCs is dependent on CD4+ T helper cells.42 Whether or not DCIR-mediated antigen uptake by human pDCs also results in MHC class I–dependent antigen presentation (cross-presentation) is currently unknown. We have performed pilot experiments in which we have loaded human pDCs with ovalbumin via DCIR-mediated uptake to evaluate cross-presentation. Ovalbumin was readily internalized after DCIR-mediated uptake. However, we did not observe any CD8+ T-cell response to these pDCs (data not shown), whereas proliferation of CD4+ T cells was readily observed. In addition, extensive mouse pDC studies have so far not been able to show cross-presentation by murine pDCs.43 Moreover, antigen uptake via murine DCIR2 into myeloid DCs has recently been shown to induce only helper T-cell responses and no MHC class I antigen presentation.4 These findings and our own data also imply that human DCIR is probably not involved in direct cross-presentation. Instead, the IFN-α pDCs produced in response to TLR ligands has been shown to promote cross-presentation of exogenous antigens by myeloid DCs.44-46
In conclusion, the data demonstrate that DCIR is a new human pDC endocytic receptor that controls TLR9-mediated IFN-α production. DCIR on pDCs mediates antigen uptake. This is the first time that targeting DCIR on human pDCs is shown to lead to efficient antigen presentation resulting in a memory T-cell response. Our findings help to understand how pDCs can exert opposing functions, such as immune activation and inhibition. Pathogens may use binding to immune inhibitory receptors, such as DCIR as immune evasion strategy, whereas endogenous ligands may bind to DCIR and thereby dampen immune activation. Future research will focus on identification of DCIR ligands to further understand its role in pDC biology and targeting studies aiming to modulate the immune response via DCIR.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ben Joosten for excellent technical assistance and Dr Alessandra Cambi for critical reading of this manuscript.
This work was supported in part by the Deutsche Forschungsgemeinschaft (grant ME 2051/2-1, F.M.-W.), the Dutch Cancer Society (grant 2004-3127, D.B.-R.), and by the NWO (ZonMw 918.66.615, G.J.A.) and 917.76.363 (I.J.M.d.V.).
Authorship
Contribution: F.M.-W. designed and performed research, analyzed and interpreted data, and wrote the manuscript; D.B.-R. designed and performed research and analyzed and interpreted data; P.J.T. designed research and contributed vital new reagents; C.J.A.P. contributed vital patient material; C.G.F. and I.J.M.d.V. designed research; G.J.A. designed research and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gosse J. Adema, Department of Tumor Immunology, NCMLS/ 278 TIL, University Medical Centre Nijmegen, PO Box 9101, 6500 HB, Nijmegen, The Netherlands; e-mail: g.adema@ncmls.ru.nl.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal