Abstract
Nanoscale imaging of an in vivo antigen-specific T-cell immune response has not been reported. Here, the combined near-field scanning optical microscopy– and fluorescent quantum dot–based nanotechnology was used to perform immunofluorescence imaging of antigen-specific T-cell receptor (TCR) response in an in vivo model of clonal T-cell expansion. The near-field scanning optical microscopy/quantum dot system provided a best-optical-resolution (<50 nm) nano-scale imaging of Vγ2Vδ2 TCR on the membrane of nonstimulated Vγ2Vδ2 T cells. Before Ag-induced clonal expansion, these nonstimulating Vγ2Vδ2 TCRs appeared to be distributed differently from their αβ TCR counterparts on the cell surface. Surprisingly, Vγ2Vδ2 TCR nanoclusters not only were formed but also sustained on the membrane during an in vivo clonal expansion of Vγ2Vδ2 T cells after phosphoantigen treatment or phosphoantigen plus mycobacterial infection. The TCR nanoclusters could array to form nanodomains or microdomains on the membrane of clonally expanded Vγ2Vδ2 T cells. Interestingly, expanded Vγ2Vδ2 T cells bearing TCR nanoclusters or nanodomains were able to rerecognize phosphoantigen and to exert better effector function. These studies provided nanoscale insight into the in vivo T-cell immune response.
Introduction
T-cell receptors (TCRs) play a crucial role in recognition of antigens and development of immune responses. Whereas immune events for TCR-mediated recognition, signaling, and activation are well described,1-4 nanoscale imaging of immunobiology of antigen-specific TCR during the in vivo clonal T-cell expansion has not been studied. Because TCRs trigger downstream signaling and activation after antigen recognition, some unique TCR nanostructures may develop after TCR contact on Ag/antigen-presenting cell (APC) and thus contribute to selected functions, such as clonal expansion, effector function, contracting (clonal exhaustion), or differentiation. Whereas this presumption can be tested by imaging or visualization of antigen-specific TCR during the in vivo T-cell response, conventional imaging techniques using fluorescent or confocal microscopy do not have nanoscale optical resolution power to reveal individual TCRs and their dynamics during clonal expansion–maturation.1-4 Nanotechology-based imaging may make it possible to reveal TCR nanostructures in the context of T-cell recognition of antigens and therefore provide new insight into T-cell response or ultimately immunity.5
Nanotechnology is emerging as a multidisciplinary tool to advance life science and medicine.6,7 However, nanoscale imaging or dissecting of functional biologic molecules in cells remain challenging. Near-field scanning optical microscopy (NSOM) has proved to be a useful nanotechnology tool for studying hard and flat materials, but its application in biomedical research is still limited.8-11 Complicated natures of cell membranes or biologic molecules make it difficult for NSOM to generate high spatial resolution images. Whereas home-made NSOM operating in liquid can yield images of biologic molecules, the current commercial NSOM instruments are all designed for in-air imaging,12-15 posing a challenge for nanoscale imaging of cell membrane proteins. Although NSOM combined with some common fluorescent materials were used for imaging,16 the absence of highly photostable fluorophores for use in NSOM is perhaps one of the major reasons why NSOM has not been reproducibly used for nanoscale imaging of functional cellular molecules.
We made use of our expertise of γδ/αβ T-cell biology17 and nanotechnology18,19 to perform nanoscale immunofluorescence imaging of antigen-specific T-cell antigen receptor (TCR) response during the in vivo clonal activation–expansion. We first established a NSOM- and fluorescent quantum dot (QD)-based imaging system to generate a best-optical-resolution immunofluorescence imaging of a cell membrane protein. We then used this powerful NSOM/QD imaging system to visualize nonengaging TCR molecules on the cell membrane of unstimulated Vγ2Vδ2 T cells and compared γδ TCR with their αβ TCR counterparts for membrane distribution patterns before activation–expansion. Based on these studies, we determined whether some unique nanostructures of TCR molecules developed during the in vivo clonal T-cell expansion. Such nanoscale imaging studies demonstrated that the high-density TCR nanoclusters not only were formed but also sustained on the membrane of clonally expanded Vγ2Vδ2 T cells and that these TCR nanoclusters were associated with the capability of clonally expanded Vγ2Vδ2 T cells to rerecognize antigen and to exert effector function during the Ag-mediated clonal expansion.
Methods
Animals
Eight cynomolgus (Macaca fascicularis) macaques, 4 to 8 years old (3-5 kg weight) were used for collection and purification of T cells from blood and assessed for in vivo Vγ2Vδ2 T-cell expansion by phosphoantigen HMBPP/interleukin-2 (IL-2) or Picostim/IL-2 treatment. Animals treated with Picostim/IL-2 were simultaneously vaccinated intradermally with 106 CFU of bacillus Calmette-Guerin, which had no impact on early response because of the intradermal route. The animal studies were approved by The University of Illinois, Chicago, Institutional Animal Care and Use Committee.
Phosphoantigen compounds: HMBPP and Picostim
The phosphoantigen compound, (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) was an analytic-pure synthetic compound with chemical structure identical to natural phosphoantigen produced from Escherichia coli or mycobacteria.20 Molecule weight of HMBPP is 262 Da. Picostim was another phophoantigen compound that shares chemical structure and bioactivity with HMBPP, except that only one atom is different, linking the carbon chain and the phosphate moiety (oxygen or carbon). These well-defined phosphoantigens were recognized by human and monkey Vγ2Vδ2 T cells.20 HMBPP and Picostim were produced, characterized, validated, and provided by Dr Hassan Jomaa from Justus-Liebig-Universität Giessen (Giessen, Germany) and the company Innate Pharma (Marseille, France), respectively.
HMBPP or Picostim treatment of monkeys
HMBPP (50 mg/kg) or Picostim (32 mg/kg) was administered to a monkey intramuscularly on day 0. One million IU of recombinant human (rh) IL-2 was given once daily from days 0 through 5 by subcutaneous injection. The HMBPP or Picostim/IL-2 treatment of monkeys reproducibly induced remarkable expansion of Vγ2Vδ2 T cells but not other γδ or αβ T-cell subpopulations.
mAbs and QD conjugates for surface staining
Cross-reactive antihuman TCR antibodies were purchased from Pierce (Rockford, IL) and Coulter (Marseille, France), as previously described.17 These monoclonal antibodies (mAbs) were as follows: fluorescein isothiocyanate (FITC)-conjugated anti–human Vγ2 (7A5, mouse IgG1), anti–human Vδ2 (15D, mouse IgG1), anti–human Vβ5a (1C1, mouse IgG1), anti–human Vβ3.1, anti–human Vβ17 (all from Pierce). All these TCR antibodies were validated for use in monkeys as we described previously.21 Goat anti–mouse IgG (Fc)-PE (for control experiments; Beckman Coulter, Fullerton, CA), APC-conjugated CD3 (SP34-2, mouse IgG1), pacific blue-conjugated CD3, FITC-conjugated anti–human CD3, goat anti–mouse IgG (H+L)-Pacific Blue (Invitrogen, Carlsbad, CA), fluorescent QD 655-conjugagted goat anti–mouse IgG conjugate (H+L; previously, Quantum Dot, Hayward, CA; presently, Invitrogen, Carlsbad, CA), fluorescent QD-conjugated streptavidin (Invitrogen), and biotinylated goat anti–mouse IgG conjugate (H+L) (Invitrogen). The QD655 were defined by Invitrogen as ellipsoid approximately 6 to 12 nm, and the addition of antibody or streptavidin complex yields a particle approximately 20 nm in diameter. The QD655-streptavidin staining protocol and reagents provided by Invitrogen appeared to favor one-on-one interaction with biotin. Both QD655-Ab and QD655-streptavidin yielded a comparable immunofluorescence imaging of individual TCR on cell surface.
Antibody staining and enrichment of Vβ5+, Vβ3.1+, Vβ17+, Vδ2+, or Vγ2+ T cells
Peripheral blood lymphocytes (PBLs) isolated freshly from the blood prospectively collected from each monkey were fixed briefly for 30 minutes by 2% formaldehyde in phosphate-buffered saline (PBS) buffer (pH 7.4) followed by 3 times wash with PBS before the staining by primary antibody: anti-Vδ2 Ab, anti-Vγ2 Ab, anti-Vβ5 Ab, anti-Vβ3.1 Ab, anti-Vβ17 Ab, or other Abs. The short-term fixing protocol allowed us to readily stain all these T-cell subpopulations without causing any activation effects (data not shown). After washing with PBS buffer, QD655-conjugated goat anti–mouse IgG was added (or biotinylated goat anti–mouse IgG and then streptavidin-conjugated QD655) followed by 2 times wash with PBS. Both primary and secondary antibody reactions were done at 4°C for 30 minutes, and washed 2 times with PBS following the standard protocol. To enrich the Ab-stained cells, cells were magnetically labeled with goat anti–mouse IgG MicroBeads (Miltenyi Biotec, Auburn, CA). Then the cell suspension was loaded onto an MS column that was placed in the magnetic field of a Mini MACS Separator. After removal of the column from the magnetic field, the magnetically retained cells were eluted as the positively selected cells. The eluted cells were then ready for confocal and NSOM imaging. This enrichment procedure gave rise to up to 50% to 70% of Vδ2+, Vγ2+, Vβ3+, Vβ17+, or Vβ5+ T cells. Such a moderate level of enrichment using a less stringent washing of the column appeared to be adequate for evaluating antibody staining specificity because NSOM can detect both the specific Ab-stained cells and those control cells (as controls) that were negatively stained but still eluted from the magnetic column. After HMBPP/IL-2 or Picostim/IL-2 treatment, there was no need to enrich Vδ2+ or Vγ2+ T cells because the number of Vγ2Vδ2 T cells increased to more than 30% to 65% in the blood within days 4 to 7 after the treatment. The immune-stained cells were fixed again with 2% formal-dehyde in PBS buffer and washed 2 times with double distilled water before NSOM imaging.
Control experiments for avoiding nonspecific antibody staining
To ensure the antibody specific staining of Vδ2+, Vγ2+, Vβ3+, Vβ17+, or Vβ5+ T cells, the following controls were always included: (1) PBLs were incubated with IgG isotype-matched control antibodies followed by QD-conjugated Ab or streptavidin, or FITC- or PE-, APC-, or Pacific blue-conjugated Ab. Flow cytometric and confocal microscopic analyses showed an absence of detectable nonspecific staining in the setting of the control experiments. (2) PBLs were incubated with QD-conjugated Ab and then magnetically labeled by a 30-minute incubation with goat anti–mouse IgG microbeads as described, and selected by the Mini MACS Separator. There was no detectable positive staining of these cells, suggesting a lack of nonspecific binding to PBLs by QD-conjugated Ab or streptavidin. In the initial experiments, CD3, CD4, or CD8 antibody staining of PBLs followed by QD-conjugated Ab was also included as positive controls to ensure that the whole staining procedure worked.
Flow cytometry
Three-color flow cytometric analyses were performed on a CyAn ADP high-performance research flow cytometer (Dako, Carpinteria, CA). Staining panel was Vγ2/Vδ2/CD3. Lymphocytes were gated by forward and side scatters, and up to 20 000 gated cells were analyzed, as previously described.
Confocal microscopy
Slides loaded with antibody-stained cells were viewed on a Carl Zeiss (Thornwood, NY) LSM510 Meta5 laser scanning confocal microscope equipped with a 63×/1.2 water-immersion objective or a 100×/1.30 oil-immersion objective. Beams of 488 nm from an Ag/Kr laser and 405 nm from a diode laser were used for excitation. Red, green, and blue fluorescence emissions were detected through LP 650, BP 505-550, BP 420-480, respectively, and other filters using a photomultiplier tube and LSM510 software (Carl Zeiss). The different fluorochromes were scanned sequentially using multitracking function to avoid any bleed-through among these fluorescent dyes.
NSOM imaging
An Aurora 3 NSOM instrument (Veeco, Santa Barbara, CA) was used for imaging. NSOM aperture probes purchased for use from Veeco were 50 nm in diameter. The defined NSOM probes were assessed for their ability to confer nanoscale images of Ab- or streptavidin-conjugated QD655 on glass substrates or the QD655-Ab-bound TCR on cell membrane. For NSOM imaging of fluorescent Ab-conjugated QD655 or streptavidin-conjugated QD655 on glass substrates, the QD655 suspension were spread on the clean glass substrates as described.11 The NSOM images of individual well-characterized QD655 on the glass served as control to estimate QD655-Ab–labeled TCR dots on cell membrane. For NSOM imaging of cells, the QD655-Ab–stained cell sample was split into 2 parts: one for confocal microscopy and the other for NSOM imaging. Each half contained 105 to 106 cells in 100 μL PBS buffer. Before NSOM imaging, confocal microscopy analysis of stained T-cell samples was performed to determine whether the cells remained healthy, whether the positive controls worked, whether negative controls (control [1] and control [2] in the control experiments) remained negative, and whether the number of the positive cells (generally, > 20% should be necessary) were enough for subsequent NSOM imaging. Before NSOM imaging, fresh coverslips were cleaned and treated with 0.1% poly-L-lysine (Sigma-Aldrich, St Louis, MO) for more than 1 hour after by washing with double-distilled water. A drop of mAb-stained cell suspension in double-distilled water was deposited onto the poly-L-lysine-coated coverslip, dried in air at 22°C for 1 hour as described,11 and then subjected to NSOM imaging in transmission mode. In the quality-control experiments, the NSOM images of immune-stained cells dried for 1 hour were compared with the images of the same cells that were dried for 1 day or 7 days. We found that the fluorescent images of QD655-bound γδ TCR in those settings were highly comparable. In Aurora 3 NSOM operation (Veeco), the continuous wave semiconductor laser (Coherent, Cube, 404 nm), NSOM probes with an aperture diameter of 50 nm (Veeco), and a 650/40 nm bandpass filter were used to excite and collect emitted fluorescence from fluorophores, which was detected by an avalanche photon detector (SPCM-AQR-14; PerkinElmer, Vaudreuil, QC).
To perform reproducible NSOM imaging, we had characterized each box of 50-nm probes and screened for the right probes that could confer NSOM images of individual Vγ2Vδ2 TCR dots (∼50 nm) on cell membrane of immune-stained γδ T cells in the blood repeatedly collected from 2 monkey donors. The well-characterized QD655 could replace the fluorescent nanospheres in our system to test or characterize the utility of NSOM probes. When γδ T cells and αβ T cells were compared for surface TCR patterns, the same probe was used to scan γδ T cells and then αβ T cells or another way around. Similarly, the same probe was used to longitudinally scan γδ T cells collected from a monkey after phosphoantigen treatment. The selected probes were able to detect various fluorescent dots ranging widely from approximately 50-nm individual TCR dots to more than 60-nm TCR dots or clusters in each of the scanned cells despite the difference in TCR patterns among the cells. To ensure that more than 60 nm TCR or TCR clusters detected in NSOM/QD system were not the result of the spontaneous oligomerization of QD, the QD655 was filtered through a 80- to 100-nm filter right before adding it to cell suspension for staining. In addition, the second Ab-conjugated QD655 was added to the cell suspension containing anti-Vγ2 (1st Ab)-bound T cells under confocal microscopy, and observed for the membrane fluorescence intensity in real time. No oligomerization of QD655 on cell membrane was seen since there was an absence of postbinding fold-increases in fluorescence intensity after the addition of QD655 (second Ab).
Intracellular interferon-γ or perforin staining
This was done as previously described.22 PBLs were stimulated using the HMBPP (10 ng/mL), stained intracellularly for interferon-γ (IFNγ) and perforin, and analyzed by flow cytometry for IFNγ or perforin-positive Vγ2Vδ2 T cells. Briefly, PMA and ionomycin (positive control) or media alone (negative control) are added to 106 peripheral blood mononuclear cells/200 μL media/tube. The cultures are incubated for 1 hour at 37°C, 5% CO2, followed by an additional 5-hour incubation in the presence of a secretion inhibitor, brefeldin A (GolgiPlug, BD Biosciences, San Diego, CA), to prevent IFNγ or perforin from being secreted from the cells. The cells were then stained for intracellular IFNγ or perforin as well as cell surface molecules of Vγ2, Vδ2, CD3. Up to 5 color staining were used to phenotype IFNγ- or perforin-producing Vγ2Vδ2 T cells. These experiments allowed us to evaluate the frequency of IFNγ- or perforin-producing Vγ2Vδ2 effector T cells in response to HMBPP phosphoantigen (HMBPP stimulates Vγ2Vδ2 T cells but not other cells).
Data processing and statistics
SPMLab6.0.2 (Veeco) was used to analyze NSOM data. Only leveling and convolution or filtering were applied to the original NSOM fluorescence images (no processing to topographic images). Both Image-Pro Plus 5.0 (Media Cybrnetics, Silver Spring, MD) and SPMLab software (Veeco) were complementarily used to measure full widths at half maximum (FWHM) and size distribution of single individual QD655-Ab on smooth glass substrates and QD655-Ab–bound TCR on cell membrane. Both less than or equal to 60 nm and more than 60 nm QD655-Ab dots on glass and QD655-Ab–TCR dots on cell membrane were automatically distinguished and counted by the Image-Plus software based on the fluorescence intensity and sizes, and then delineated in teal. Complementarily, intensity distribution of fluorescent QD655-Ab on the glass substrates and fluorescent QD655-Ab–bound TCR on the cell membrane of Vγ2Vδ2 T cells was also measured using Image-Pro Plus 5.0. Because fluorescent size (FWHM) frequency corresponded well to intensity distribution in the settings of both the individual QD655-Ab on the glass and the QD655-Ab–TCR on the cell membrane, measurements of sizes of dots on cell membrane appeared to be useful for detecting and counting TCR dots and clusters. TCR dots and clusters were further measured and counted using the NSOM SPMLab software-based quantitation. To do this, the diameters of FWHM of all TCR clusters with various sizes were measured and quantitated based on the fluorescence intensity profiles of the cross sections crossing the centers of these clusters. EasyFit 1.3 (MathWave Technologies, Dnipropetrovsk, Ukraine) was used to make histograms displaying frequencies of TCR dots and clusters. Adobe Photo CS2 was applied to edit the images. Confocal microscopic images were browsed by Zeiss LSM Image Browser (Carl Zeiss). Statistic analyses were done using Student t test to determine the biologic significance for the values between αβ T cells and γδ T cells.
Results
A NSOM- and QD-based system conferred nanoscale immunofluorescence imaging of nonengaging Vγ2Vδ2 TCR on cell membrane
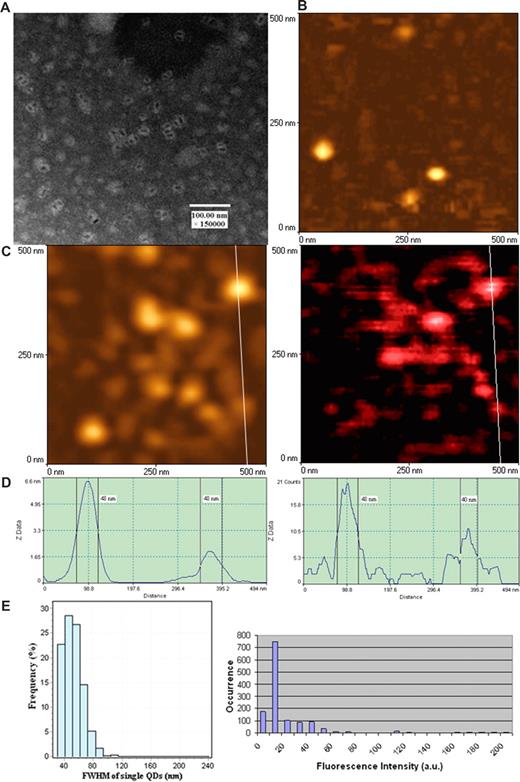
To perform nanoscale immunofluorescence imaging of antigen-specific TCR response, we took advantage of unique optical qualities of fluorescent QD23-32 and used this innovative fluorescent material for NSOM imaging. Initial efforts were made to ensure that NSOM probes defined by the vendor as 50 nm in diameters were able to confer nanoscale images of the defined antibody (Ab)- and streptavidin-conjugated QD (see “mAbs and QD conjugates for surface staining” and “NSOM imaging”). QD655 used as fluorochrome in our experiments exhibited as individual dots with a diameter of approximately 10 nm under EM (Figure 1A). The NSOM topographic imaging showed that individual Ab-conjugated QD655 were approximately 50 nm in diameters resulting from the convolution effect of the probe (left panels of Figure 1C,D). The NSOM fluorescence imaging showed that the FWHM of individual QD655 were approximately 50, with 50-nm QDs being predominant (right panels of Figure 1C,D). The predominance of approximately 50-nm fluorescence dots corresponded to the dominant intensity distribution of individual fluorescent QD655 dots on the same glass substrate (Figure 1E). QDs with 20 fluorescence counts were dominant and the smallest ones among all the QDs on the glass substrate, which was consistent with the 40- to 50-nm FWHM dominance (Figure 1E). Thus, these results demonstrated that the majority of QD655 molecules with 20 fluorescence counts were those fluorescence dots with a size of approximately 50 nm.
Combined aperture NSOM and fluorescent QD nanotechnology conferred approximately 50-nm resolution scale fluorescence imaging of individual QD655 on glass substrate. (A) Electron micrograph of fluorescent quantum dots (QD655). These disperse semiconductor nanocrystals have a diameter of approximately 10 nm under EM. The EM image was derived from a JEOL JEM-1220 transmission electron microscope using a standard EM protocol. (B) NSOM topographic image of the surface of clean glass substrates (no detectable fluorescence; data not shown). (C) NSOM topographic (left) and corresponding fluorescence (right) images of individual Ab-conjugated QD655 molecules. Scan size: 500 nm × 500 nm. Resolution: 300 pixel × 300 pixel. Integration time: 10 ms. (D) The height (left) and fluorescence intensity (right) profiles of the cross sections corresponding to the white lines on images of panel C. (C,D) The diameters of individual Ab-conjugated QD655 are predominantly approximately 50 nm both in topography and fluorescence intensity, which is consistent with that of individual quantum dots bound to TCR on cell membrane surface as shown in Figure 2. Similar results were seen for streptavidin-conjugated fluorescent QD (data not shown). (E) The left histogram shows the frequency or distribution for the immunofluorescence FWHM of individual Ab-conjugated QD655 molecules (n = 608); the right histogram shows fluorescence intensity distribution of Ab-conjugated QD655 on the same glass substrate. Mean diameters of the individual conjugated QD655 were 51.63 plus or minus 13.46 nm (mean ± SD), with approximately 50-nm dots being predominant (left histogram); the scale 20 was the dominant fluorescent intensity unit of the individual conjugated QD655 on the glass substrate (right histogram). The scale 20 was the weakest fluorescence intensity counts, even on the glass substrate prepared from further dilutions of QDs (≤ 10 intensity scales are background).
Combined aperture NSOM and fluorescent QD nanotechnology conferred approximately 50-nm resolution scale fluorescence imaging of individual QD655 on glass substrate. (A) Electron micrograph of fluorescent quantum dots (QD655). These disperse semiconductor nanocrystals have a diameter of approximately 10 nm under EM. The EM image was derived from a JEOL JEM-1220 transmission electron microscope using a standard EM protocol. (B) NSOM topographic image of the surface of clean glass substrates (no detectable fluorescence; data not shown). (C) NSOM topographic (left) and corresponding fluorescence (right) images of individual Ab-conjugated QD655 molecules. Scan size: 500 nm × 500 nm. Resolution: 300 pixel × 300 pixel. Integration time: 10 ms. (D) The height (left) and fluorescence intensity (right) profiles of the cross sections corresponding to the white lines on images of panel C. (C,D) The diameters of individual Ab-conjugated QD655 are predominantly approximately 50 nm both in topography and fluorescence intensity, which is consistent with that of individual quantum dots bound to TCR on cell membrane surface as shown in Figure 2. Similar results were seen for streptavidin-conjugated fluorescent QD (data not shown). (E) The left histogram shows the frequency or distribution for the immunofluorescence FWHM of individual Ab-conjugated QD655 molecules (n = 608); the right histogram shows fluorescence intensity distribution of Ab-conjugated QD655 on the same glass substrate. Mean diameters of the individual conjugated QD655 were 51.63 plus or minus 13.46 nm (mean ± SD), with approximately 50-nm dots being predominant (left histogram); the scale 20 was the dominant fluorescent intensity unit of the individual conjugated QD655 on the glass substrate (right histogram). The scale 20 was the weakest fluorescence intensity counts, even on the glass substrate prepared from further dilutions of QDs (≤ 10 intensity scales are background).
We also found that QDs were more reproducible for NSOM imaging than other traditional fluorochromes. Ab- or streptavidin-conjugated QD, but not FITC, PE, APC, Pacific blue, or Cy5, were photostable or resistant to photobleaching on laser excitation (data not shown). Actually, Ab-conjugated QD655 bound to cell membrane could maintain strong fluorescence after exposure in air for more than a week and repeatedly be imaged by NSOM. These findings indicated that the nanoscale, photostable, and long-lasting features of Ab- or streptavidin-conjugated QDs were well suited for an NSOM-based imaging.
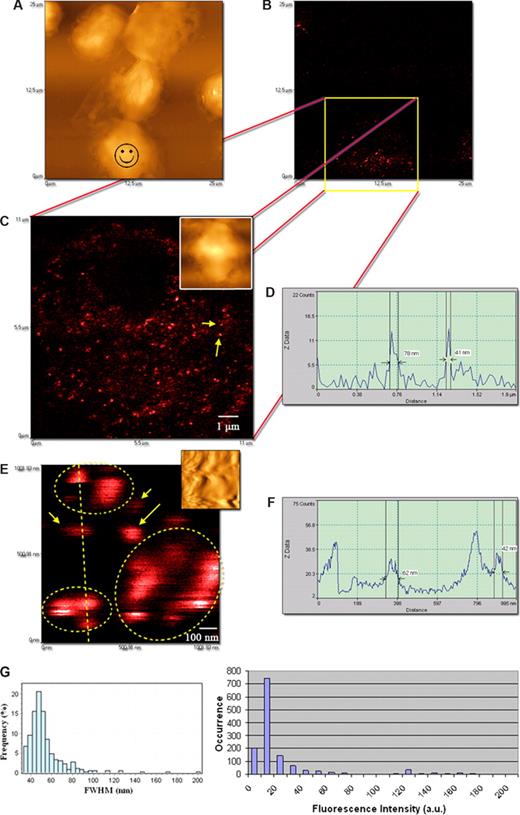
We then performed the NSOM- and QD-based imaging of nonengaging γδ TCR on the membrane of nonstimulated Vγ2Vδ2 T cells from monkeys. Phosphoantigen-specific Vγ2Vδ2 T cells constitute 60% to 95% human circulating γδ T cells, and our earlier work has demonstrated that macaque Vγ2Vδ2 T cells can mount major clonal expansion and antimycobacterial responses.17 The other reason for using this model is that Vγ2Vδ2 TCR recognize phosphoantigens, including HMBPP,33,34 and anti-Vγ2 mAb can block HMBPP-mediated activation–expansion (data not shown). The NSOM/QD system conferred a nanoscale immunofluorescence imaging of many individual nonengaging γδ TCR dots on cell membrane (Figure 2). The approximately 50-nm QD655-Ab-bound TCR dots were separated from each other and dominated on the membrane of nonstimulated Vγ2Vδ2 T cells (Figure 2C-F). Similar to what was seen for QDs on the glass substrate (Figure 1E), QD-655–bound TCR dots that exhibited 20 fluorescence counts were dominant and weakest in intensity among all the QD-bound TCR dots on the cell surface (Figure 2G). The dominance of QDs with 20 fluorescence counts corresponded to the dominance of approximately 50-nm FWHM TCR when the intensity distribution of the individual TCR dots was analyzed in comparisons with their size distribution (Figure 2G). An apparent relationship for FWHM data and intensity distribution between the QD655-Ab on glass substrate and QD655-Ab-bound TCR on cell membrane under the same excitation condition suggested that approximately 50-nm immunofluorescence TCR dots on the membrane likely represented basic TCR complex that are detectable using NSOM/QD nanotechnology. Notably, some fluorescent spots were less than 50 nm, instead of 50 nm on cell membrane. This might result from blinking property of fluorescent QD or NSOM convolution effects of contours and fluorescence of various degrees because of the height of QD655 (see “mAbs and QD conjugates for surface staining”). However, the data of fluorescent size and intensity distribution suggested that they were parts of the immune fluorescence images of Vγ2Vδ2 TCR (Figures 1E,2G). On the other hand, there were detectable fluorescent TCR clusters that were approximately 2-, 3-, 4-, or 5-fold bigger than 40- to 60-nm TCR dots (Figures 2G,3C). These fold-increasing immunoflu-orescence TCR clusters may correspond to TCR complexes comprising of multiple TCR molecules of various amounts (Figure 3C).
The NSOM/QD-based system conferred approximately 50-nm resolution scale fluorescence imaging of QD-bound γδ TCR on cell membrane of nonstimulated Vδ2 T cells. (A) Low-magnification NSOM topographic and (B) fluorescence images of 7 nonstimulated blood lymphocytes. Vδ2 TCR+ T cells are indicated with a smiley face. (C) The high-magnification NSOM fluorescence and topographic (inset) images of the cell marked by a smiley face as shown in panel A. (D) The fluorescence intensity profile of the cross section between the 2 fluorescent objects marked by 2 arrows in panel C. The short arrow shows an approximately 41-nm fluorescence dot representing a 1-QD–bound TCR dot, and the long arrow shows an approximately 80-nm fluorescence dot possibly corresponding to 2-QD-bound TCR cluster. (E) The NSOM fluorescence images enlarged from an area in panel C (inset: the corresponding topographic image). The higher-magnification imaging shows immunofluorescence images of individual 1× approximately 50-nm TCR dots (short arrows), 2× approximately 50-nm TCR cluster (long arrow), and more than 2× approximately 50-nm TCR clusters (dotted circles). (F) The fluorescent intensity profile of the cross section of the objects marked by a dashed line in panel E. Scan size: (C) 11 × 11 μm2; (E) approximately 1 × 1 μm2. Resolution: (C) 500 × 500 pixel2; (E) 400 × 400 pixel2. Integration time: (C) 15 ms; (E) 10 ms. (G) The left histogram showing the frequency or distribution for the immunofluorescence FWHM of individual Ab-QD–bound TCR (n = 326) on cell membrane of cells; the right histogram showing fluorescence intensity distribution of Ab-QD–bound TCR on the same cells. Mean FWHM diameters of the QD-Ab-bound TCR were 53.7 plus or minus 18.9 nm (mean ± SD), with approximately 50-nm dots being predominant (left histogram); the scale 20 was the dominant fluorescent intensity count of the individual QD-Ab-bound TCR on the cell membrane of cells (right histogram). Similar to what is seen in Figure 1E, individual QD-Ab–bound TCR dots exhibited a good relationship between approximately 50-nm dots and 20 intensity counts (basic-scale fluorescence) on the cell membrane (< 10 intensity scales were background). The excitation condition was the same as that described in Figure 1E. Scan size: 500 nm × 500 nm. Resolution: 300 pixel × 300 pixel. Integration time: 10 ms. The imaging was derived from Ab-conjugated QD655 as the second Ab. Similar results were seen when using QD655-streptavidin.
The NSOM/QD-based system conferred approximately 50-nm resolution scale fluorescence imaging of QD-bound γδ TCR on cell membrane of nonstimulated Vδ2 T cells. (A) Low-magnification NSOM topographic and (B) fluorescence images of 7 nonstimulated blood lymphocytes. Vδ2 TCR+ T cells are indicated with a smiley face. (C) The high-magnification NSOM fluorescence and topographic (inset) images of the cell marked by a smiley face as shown in panel A. (D) The fluorescence intensity profile of the cross section between the 2 fluorescent objects marked by 2 arrows in panel C. The short arrow shows an approximately 41-nm fluorescence dot representing a 1-QD–bound TCR dot, and the long arrow shows an approximately 80-nm fluorescence dot possibly corresponding to 2-QD-bound TCR cluster. (E) The NSOM fluorescence images enlarged from an area in panel C (inset: the corresponding topographic image). The higher-magnification imaging shows immunofluorescence images of individual 1× approximately 50-nm TCR dots (short arrows), 2× approximately 50-nm TCR cluster (long arrow), and more than 2× approximately 50-nm TCR clusters (dotted circles). (F) The fluorescent intensity profile of the cross section of the objects marked by a dashed line in panel E. Scan size: (C) 11 × 11 μm2; (E) approximately 1 × 1 μm2. Resolution: (C) 500 × 500 pixel2; (E) 400 × 400 pixel2. Integration time: (C) 15 ms; (E) 10 ms. (G) The left histogram showing the frequency or distribution for the immunofluorescence FWHM of individual Ab-QD–bound TCR (n = 326) on cell membrane of cells; the right histogram showing fluorescence intensity distribution of Ab-QD–bound TCR on the same cells. Mean FWHM diameters of the QD-Ab-bound TCR were 53.7 plus or minus 18.9 nm (mean ± SD), with approximately 50-nm dots being predominant (left histogram); the scale 20 was the dominant fluorescent intensity count of the individual QD-Ab-bound TCR on the cell membrane of cells (right histogram). Similar to what is seen in Figure 1E, individual QD-Ab–bound TCR dots exhibited a good relationship between approximately 50-nm dots and 20 intensity counts (basic-scale fluorescence) on the cell membrane (< 10 intensity scales were background). The excitation condition was the same as that described in Figure 1E. Scan size: 500 nm × 500 nm. Resolution: 300 pixel × 300 pixel. Integration time: 10 ms. The imaging was derived from Ab-conjugated QD655 as the second Ab. Similar results were seen when using QD655-streptavidin.
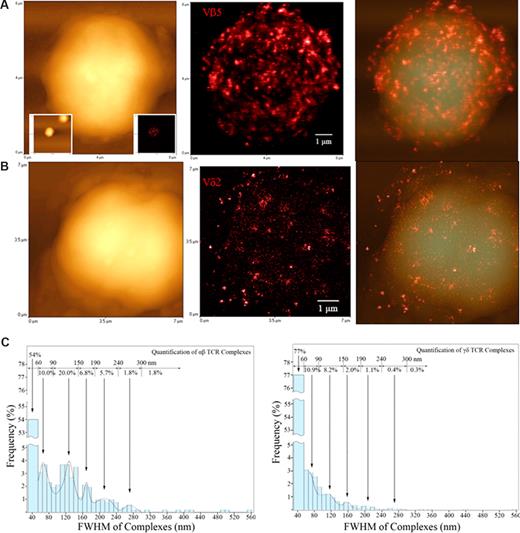
Before Ag-induced clonal expansion, nonengaging γδ TCR on nonstimulated T cells appeared to be distributed differently from their αβ TCR counterparts. Figures show topographic (left), fluorescence (middle), and merged topographic-fluorescence (right) NSOM images of αβ TCR on Vβ5+T cells (A) and γδ TCR on Vδ2+T cells (B), respectively. Note the Vβ5+T cell displaying the immunofluorescence TCR was derived from one of the 2 scanned Vβ5 cells as shown in the 2 lower-magnification graphs (25 × 25 μm2) inserted in the lower left (topographic) and lower right (fluorescence) bottom, respectively. See Figure 5B for NSOM images of a fluorescent Vγ2+ T cell and Figure S1 for more Vβ5+ and Vδ2+ T cells, and Vβ3.1+, and Vβ17+ αβ T cells. Scan size: (A) 8 × 8 μm2; (B) 7 × 7 μm2. Resolution: (A,B) 500 × 500 pixel2. Integration time: (A) 30 ms; (B) 10 ms. (C) The histograms showing a frequency difference in immunofluorescence TCR (size distribution) between the αβ T cell (left) and γδ T cell (right) using the Image-Plus software-based analysis. The data are the means calculated from up to 5 Vβ5+ cells (n = 1314) and 5 Vδ2+ cells (n = 5153). γδ T cells had more approximately 50-nm TCR dots distributed on cell surface than αβ T cells (P < .05).
Before Ag-induced clonal expansion, nonengaging γδ TCR on nonstimulated T cells appeared to be distributed differently from their αβ TCR counterparts. Figures show topographic (left), fluorescence (middle), and merged topographic-fluorescence (right) NSOM images of αβ TCR on Vβ5+T cells (A) and γδ TCR on Vδ2+T cells (B), respectively. Note the Vβ5+T cell displaying the immunofluorescence TCR was derived from one of the 2 scanned Vβ5 cells as shown in the 2 lower-magnification graphs (25 × 25 μm2) inserted in the lower left (topographic) and lower right (fluorescence) bottom, respectively. See Figure 5B for NSOM images of a fluorescent Vγ2+ T cell and Figure S1 for more Vβ5+ and Vδ2+ T cells, and Vβ3.1+, and Vβ17+ αβ T cells. Scan size: (A) 8 × 8 μm2; (B) 7 × 7 μm2. Resolution: (A,B) 500 × 500 pixel2. Integration time: (A) 30 ms; (B) 10 ms. (C) The histograms showing a frequency difference in immunofluorescence TCR (size distribution) between the αβ T cell (left) and γδ T cell (right) using the Image-Plus software-based analysis. The data are the means calculated from up to 5 Vβ5+ cells (n = 1314) and 5 Vδ2+ cells (n = 5153). γδ T cells had more approximately 50-nm TCR dots distributed on cell surface than αβ T cells (P < .05).
Before Ag-induced clonal expansion, nonengaging Vγ2Vδ2 TCR appeared to be distributed differently from nonengaging αβ TCR on cell membrane
Since Vγ2Vδ2 T cells are able to mount longer clonal expansion than CD4 or CD8 T cells in early infection,17 we used the NSOM/QD system to examine any difference in distribution patterns of cell membrane TCR between Vγ2Vδ2 T cells and their αβ T-cell counterparts before Ag-induced clonal expansion. Interestingly, a difference in distribution patterns of TCR on the cell surface was seen between Vγ2Vδ2 T cells and various Vβ-expressing αβ T cells (Figures 3,5B, and S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). A glimpse of images indicated that large TCR dots were dominant on αβ T cells, whereas approximately 50-nm γδ TCR were predominant on γδ T cells (Figures 2,3,5B,S1). Computer-based analyses showed that 40 to 60 nm (∼80%) and 60 to 90 nm (∼10%) small γδ TCR dots were predominantly distributed on cell surface, whereas αβ T cells expressed more preexisting large TCR dots (∼40%) that were more than 90 nm FWHM than γδ T cells (Figure 3). Such a difference was statistically significant (P < .05).
TCR nanoclusters were formed and sustained during an in vivo expansion of Vγ2Vδ2 T cells after phosphoantigen HMBPP/IL-2 or Picostim/IL-2 treatment
We then sought to explore dynamic changes in TCRs at nanoscale over time after the clonal expansion of Vγ2Vδ2 T cells. From immunologic standpoints, once TCRs contact Ag/APC and trigger signaling for activation, immune engagement of TCR molecules for forming TCR nanostructures on the membrane may take place and sustain to make clonally expanded T cells more competent for sensing antigen again or attacking antigen-expressing targets (infected cells) in infection. This scenario has not been addressed because of the limited optical resolution of conventional fluorescent or confocal microscopy. A combined use of confocal microscopy and NSOM/QD may make it possible to address this immunologic question. We have recently demonstrated that macaque Vγ2Vδ2 T cells can undergo major clonal expansion in response to mycobacterial infections as well as a combined phosphoantigen compound HMBPP and IL-2 treatment.17 We therefore used the in vivo model of Vγ2Vδ2 T-cell activation/expansion and the combination of confocal microscopy and NSOM/QD system to dissect the immunobiology of Vγ2Vδ2 TCR during clonal Vγ2Vδ2 T-cell expansion.
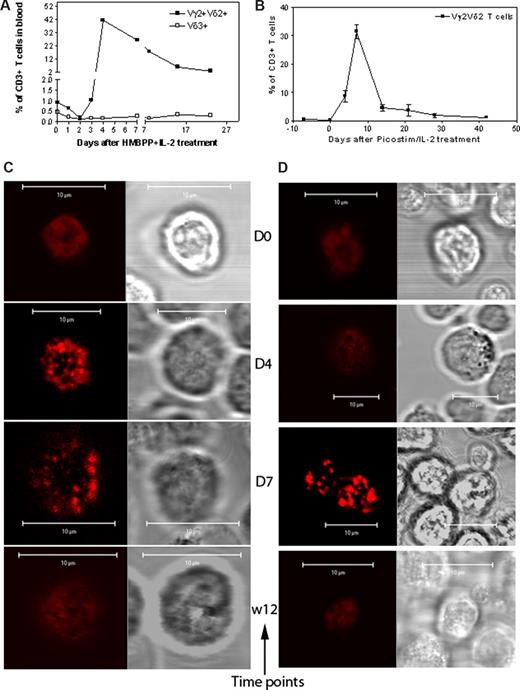
The HMBPP + IL-2 treatment reproducibly induced remarkable expansion of macaque Vγ2Vδ2 T cells in the blood circulation (Figure 4A); Picostim (almost identical to HMBPP) + IL-2 treatment similarly resulted in major expansion of Vγ2Vδ2 T cells as well (Figure 4B). The confocal microscopy showed that, whereas all the Vγ2Vδ2 T cells examined on days 4 and 7 after the HMBPP/IL-2 or Picostim/IL-2 treatment exhibited increased density of Vγ2Vδ2 TCR in the plasma membrane, the high-density TCR were displayed as aggregates or capped dots due to the limited resolution of confocal microscopy (Figures 4C,D,S2,S3).
Confocal microscopy showed that Vγ2Vδ2 TCR underwent aggregating or capping during the in vivo clonal expansion of Vγ2Vδ2 T cells after the phosphoantigen treatment. (A,B) The increased mean numbers of Vγ2Vδ2 T cells in the blood of 3 monkeys after HMBPP/IL-2 treatment (A) and 2 monkeys after Picostim/IL2 treatment (B). Almost all expanded γδ T cells were Vγ2Vδ2 T cells coexpressing both Vγ2 and Vδ2. (C,D) Representative confocal microscopic data that show the formation of aggregating and capping TCR on the clonally expanded Vγ2Vδ2 T cells at days 4 and 7 after HMBPP/IL-2 (C) or Picostim/IL-2 (D) treatment. In both panels C and D, the left panel shows fluorescence images and the right panel shows differential interference contrast. All clonally expanded Vγ2Vδ2 T cells examined on days 4 and 7 after the treatment exhibited indistinguishable large TCR aggregates or capped dots, sustained for 1 to 2 weeks, and then finally returned to the normal nonaggregated status at week 3 after the treatment (detailed data, see Figures S2,S3).
Confocal microscopy showed that Vγ2Vδ2 TCR underwent aggregating or capping during the in vivo clonal expansion of Vγ2Vδ2 T cells after the phosphoantigen treatment. (A,B) The increased mean numbers of Vγ2Vδ2 T cells in the blood of 3 monkeys after HMBPP/IL-2 treatment (A) and 2 monkeys after Picostim/IL2 treatment (B). Almost all expanded γδ T cells were Vγ2Vδ2 T cells coexpressing both Vγ2 and Vδ2. (C,D) Representative confocal microscopic data that show the formation of aggregating and capping TCR on the clonally expanded Vγ2Vδ2 T cells at days 4 and 7 after HMBPP/IL-2 (C) or Picostim/IL-2 (D) treatment. In both panels C and D, the left panel shows fluorescence images and the right panel shows differential interference contrast. All clonally expanded Vγ2Vδ2 T cells examined on days 4 and 7 after the treatment exhibited indistinguishable large TCR aggregates or capped dots, sustained for 1 to 2 weeks, and then finally returned to the normal nonaggregated status at week 3 after the treatment (detailed data, see Figures S2,S3).
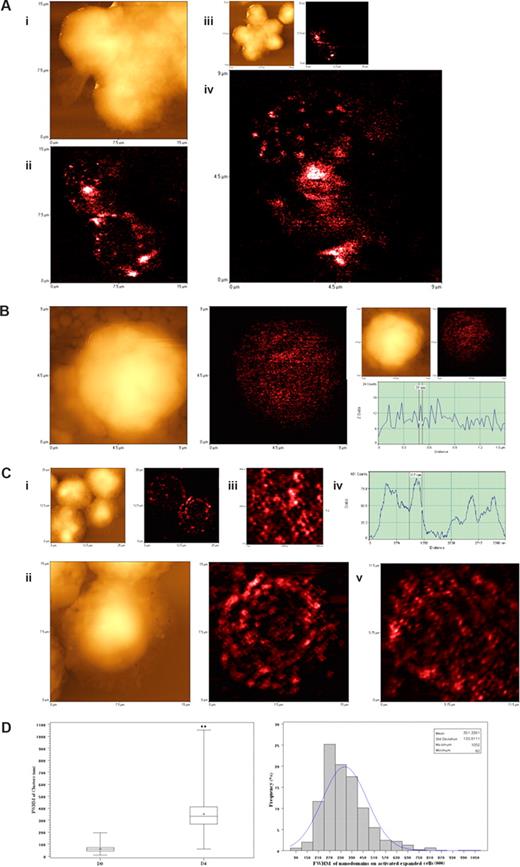
Since confocal microscopy was not able to reveal a high-resolution image of the activation-related TCR aggregates, we used the NSOM/QD imaging system to dissect the aggregated or capped TCR of clonally expanded Vγ2Vδ2 T cells. We focused on the TCR nanostructures of clonally expanded Vγ2Vδ2 T cells in PBLs collected on days 4 and 7 after the HMBPP/IL-2 or Picostim/IL-2 treatment because all the Vγ2Vδ2 T cells examined at these time points exhibited TCR aggregates on the cell membrane under confocal microscopy (Figures 4C,D,S2,S3). To facilitate evaluation of high-density Vγ2Vδ2 TCR, we arbitrarily defined 3 scales of TCR nanostructures based on the nano concept (nanomedicine dealing with ≤ 250 nm measurements) and immunofluorescence sizes of Vγ2Vδ2 TCR: (1) nanoclusters less than or equal to 250 nm, (2) nanodomains more than 250 nm but less than 1000 nm, and (3) microdomains more than or equal to 1000 nm. Interestingly, well-arrayed TCR clusters were formed and dominated on the cell membrane of the clonally expanded Vγ2Vδ2 T cells on day 4 after HMBPP/IL-2 treatment (Figure 5A-C). This was contrasted to the dominance of approximately 50-nm TCR dots on the membrane of nonstimulated Vγ2Vδ2 T cells (Figures 2,3,5B). The computer-based quantitative analyses of fluorescent dots indicated that, whereas TCR nanoclusters (≤ 250 nm) and nanodomains (> 250 nm) were readily seen, 270- to 390-nm TCR nanodomains were most frequent among the detectable TCR clusters on the membrane of the clonally expanded Vγ2Vδ2 T cells after the HMBPP/IL-2 treatment (Figure 5A,C). The TCR nanoclusters were also congregated to form many larger nanodomains ranging from 450 nm to 810 nm and a few microdomains with sizes more than 1000 nm (Figure 5A,C). Similarly, high-density TCR nanoclusters, nanodomains, and microdomains were also seen on the membrane of clonally expanded Vγ2Vδ2 T cells on days 4 and 7 after the phosphoantigen Picostim/IL-2 treatment (Figure 6). The high-density TCR nanoclusters/nanodomains revealed by the NSOM/QD system of clonally expanded Vγ2Vδ2 T cells appeared to be the detailed nanographs dissecting those undistinguished TCR aggregates or cappings as seen vaguely under confocal microscopy (Figures 4B,S2,S3). Therefore, these findings demonstrated that high-density TCR nanoclusters not only were formed but also sustained on the membrane of clonally expanded Vγ2Vδ2 T cells after the phosphoantigen/IL-2 treatment.
NSOM/QD-based imaging showed that Vγ2Vδ2 TCR arrayed to form high-density TCR nanoclusters, nanodomains, and microdomains during the in vivo clonal expansion of Vγ2Vδ2 T cells after HMBPP/IL-2 treatment. (A) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 4. (Ai) NSOM topographic images of 3 closely adjacent cells as marked by a smiley face in the low-magnification images showing 6 closely adjacent cells in panel Aiii. (Aii) The NSOM fluorescence image displaying the polarized γδ TCR nanoclusters, nanodomains, and microdomains on the 2 corresponding Vδ2 T cells as shown in panel Ai. (Aiv) The enlarged NSOM fluorescence image of TCR nanoclusters, nanodomains, and microdomains on the Vδ2 T cell shown in the upper left of panel Aii. (B) Representative NSOM topographic (left) and fluorescence (middle) images indicating the dominance of nonengaging fluorescence TCR dots on the membrane of unstimulated Vγ2 T cells on day 0. The fluorescent intensity profile graph (right) is extracted from a random cross section in the fluorescence image (middle) showing that predominant fluorescence TCR dots here displayed FWHM of approximately 50 nm. (C) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vγ2 T cells on day 4. (Cii) Enlarged from the boxed area in the low-magnification NSOM image panel Ci. The fluorescence intensity profile (Civ) is extracted from the cross-sectional part (dashed arrow in Ciii), which is enlarged from the boxed area in panel Cii. (Cv) The NSOM fluorescence image of another activated/expanded Vγ2 T cell collected on day 4. (D) Boxplot (left) and histogram (right) showing the FWHM of approximately 50-nm TCR dots and TCR nanoclusters/nanodamains/microdomains before and day 4 after HMBPP/IL-2 treatment. (**P < .001 vs the values of the unstimulated cells). In the left panel, the center line is the median, the dot is the mean, the boxes are interquartile ranges, and the whiskers are the value ranges. The histogram on the right shows the percentages of different sizes of TCR clusters in total γδ TCR that were counted and measured. The data are the mean frequencies calculated from 5 Vδ2+ and Vγ2+ T cells. Scan size: (Aiii, Ci) 25 × 25 μm2; (Ai,ii, Cii) 15 × 15 μm2; (Cv) 11.5 × 11.5 μm2; (Aiv, B) 9 × 9 μm2; (Ciii) 4.5 × 4.5 μm2. Resolution: (Aiii, Ci) 300 × 300 pixel2; (Ai, Aii, B) 400 × 400 pixel2; (Aiv, Cii,iii, and Cv) 500 × 500 pixel2. Integration time (ms): (Aii-iv) 40; (B) 15; (Ci-iii) 10; (Cv) 20.
NSOM/QD-based imaging showed that Vγ2Vδ2 TCR arrayed to form high-density TCR nanoclusters, nanodomains, and microdomains during the in vivo clonal expansion of Vγ2Vδ2 T cells after HMBPP/IL-2 treatment. (A) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 4. (Ai) NSOM topographic images of 3 closely adjacent cells as marked by a smiley face in the low-magnification images showing 6 closely adjacent cells in panel Aiii. (Aii) The NSOM fluorescence image displaying the polarized γδ TCR nanoclusters, nanodomains, and microdomains on the 2 corresponding Vδ2 T cells as shown in panel Ai. (Aiv) The enlarged NSOM fluorescence image of TCR nanoclusters, nanodomains, and microdomains on the Vδ2 T cell shown in the upper left of panel Aii. (B) Representative NSOM topographic (left) and fluorescence (middle) images indicating the dominance of nonengaging fluorescence TCR dots on the membrane of unstimulated Vγ2 T cells on day 0. The fluorescent intensity profile graph (right) is extracted from a random cross section in the fluorescence image (middle) showing that predominant fluorescence TCR dots here displayed FWHM of approximately 50 nm. (C) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vγ2 T cells on day 4. (Cii) Enlarged from the boxed area in the low-magnification NSOM image panel Ci. The fluorescence intensity profile (Civ) is extracted from the cross-sectional part (dashed arrow in Ciii), which is enlarged from the boxed area in panel Cii. (Cv) The NSOM fluorescence image of another activated/expanded Vγ2 T cell collected on day 4. (D) Boxplot (left) and histogram (right) showing the FWHM of approximately 50-nm TCR dots and TCR nanoclusters/nanodamains/microdomains before and day 4 after HMBPP/IL-2 treatment. (**P < .001 vs the values of the unstimulated cells). In the left panel, the center line is the median, the dot is the mean, the boxes are interquartile ranges, and the whiskers are the value ranges. The histogram on the right shows the percentages of different sizes of TCR clusters in total γδ TCR that were counted and measured. The data are the mean frequencies calculated from 5 Vδ2+ and Vγ2+ T cells. Scan size: (Aiii, Ci) 25 × 25 μm2; (Ai,ii, Cii) 15 × 15 μm2; (Cv) 11.5 × 11.5 μm2; (Aiv, B) 9 × 9 μm2; (Ciii) 4.5 × 4.5 μm2. Resolution: (Aiii, Ci) 300 × 300 pixel2; (Ai, Aii, B) 400 × 400 pixel2; (Aiv, Cii,iii, and Cv) 500 × 500 pixel2. Integration time (ms): (Aii-iv) 40; (B) 15; (Ci-iii) 10; (Cv) 20.
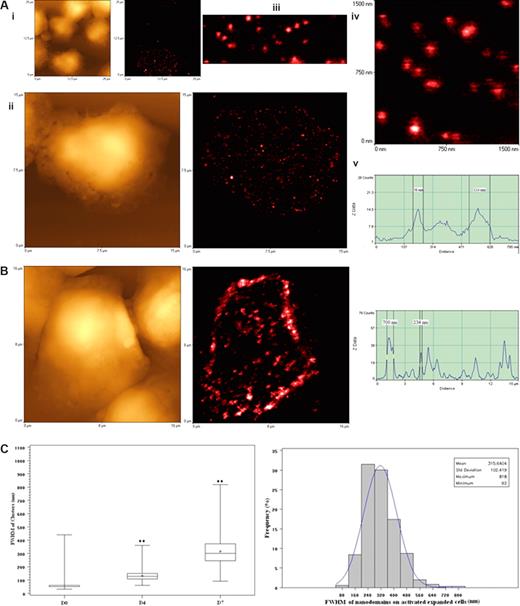
High-density TCR nanoclusters, nanodomains, and microdomains were also developed during the in vivo clonal expansion of Vγ2Vδ2 T cells after the Picostim/IL-2 treatment. (A) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 4. On the left are topographic images; on the right are fluorescent images. The images in panel Ai-Aiv are sequential magnification images from the boxed area in the image. The mean clusters on the cells at this time point are 132.7 plus or minus 37.1 nm, a nanoscale imaging that cannot be reached by confocal microscopy because of the limited optical resolution (∼300 nm). Scanning sizes: (Ai) 25 × 25 μm2; 300 × 300 pixel2; (Aii) 15 × 15 μm2; 500 × 500 pixel2; (Aiv) 1.5 × 1.5 μm2; 300 × 300 pixel2. (Av) Fluorescent intensity profile of the cross section of the objects (the (dashed line in panel Aiv). Shown are 2 TCR clusters with diameters (FWHM) of 56 nm and 114 nm, respectively. Integration time in panels Ai to Aiv: 30 ms. (B) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 7 after Picostim/IL-2 treatment. Shown are the NSOM topographic (left) and fluorescence (middle) images (16 × 16 μm2; 500 × 500 pixel2; integration time: 30 ms) of a Vγ2Vδ2 T cell. The TCR clusters on the cells at this time point are 315.6 plus or minus 102.4 nm. The fluorescent intensity profile (right) of the cross section of the objects is marked by a line in the fluorescence image (middle). Shown are 2 TCR clusters with diameters of 234 nm and 700 nm (FWHM), respectively. (C) Boxplot (left) and histogram (right) graphs showing the FWHM of approximately 50-nm TCR dots and TCR nanoclusters/nanodamains/microdomains before and days 4 and 7 after Picostim/IL-2 treatment. Box and whisker plot is as described in Figure 5D. (**P < .001 vs the values of the unstimulated cells.) The histogram on the right shows the percentages of different sizes of TCR clusters in total γδ TCR that were counted and measured. The data are the mean frequencies calculated from 5 Vδ2+ and Vγ2+ cells.
High-density TCR nanoclusters, nanodomains, and microdomains were also developed during the in vivo clonal expansion of Vγ2Vδ2 T cells after the Picostim/IL-2 treatment. (A) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 4. On the left are topographic images; on the right are fluorescent images. The images in panel Ai-Aiv are sequential magnification images from the boxed area in the image. The mean clusters on the cells at this time point are 132.7 plus or minus 37.1 nm, a nanoscale imaging that cannot be reached by confocal microscopy because of the limited optical resolution (∼300 nm). Scanning sizes: (Ai) 25 × 25 μm2; 300 × 300 pixel2; (Aii) 15 × 15 μm2; 500 × 500 pixel2; (Aiv) 1.5 × 1.5 μm2; 300 × 300 pixel2. (Av) Fluorescent intensity profile of the cross section of the objects (the (dashed line in panel Aiv). Shown are 2 TCR clusters with diameters (FWHM) of 56 nm and 114 nm, respectively. Integration time in panels Ai to Aiv: 30 ms. (B) Representative NSOM images of TCR nanoclusters, nanodomains, and microdomains on the membrane of clonally expanded Vδ2 T cells on day 7 after Picostim/IL-2 treatment. Shown are the NSOM topographic (left) and fluorescence (middle) images (16 × 16 μm2; 500 × 500 pixel2; integration time: 30 ms) of a Vγ2Vδ2 T cell. The TCR clusters on the cells at this time point are 315.6 plus or minus 102.4 nm. The fluorescent intensity profile (right) of the cross section of the objects is marked by a line in the fluorescence image (middle). Shown are 2 TCR clusters with diameters of 234 nm and 700 nm (FWHM), respectively. (C) Boxplot (left) and histogram (right) graphs showing the FWHM of approximately 50-nm TCR dots and TCR nanoclusters/nanodamains/microdomains before and days 4 and 7 after Picostim/IL-2 treatment. Box and whisker plot is as described in Figure 5D. (**P < .001 vs the values of the unstimulated cells.) The histogram on the right shows the percentages of different sizes of TCR clusters in total γδ TCR that were counted and measured. The data are the mean frequencies calculated from 5 Vδ2+ and Vγ2+ cells.
Clonally expanded Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were able to rerecognize antigen and to exert better effector function
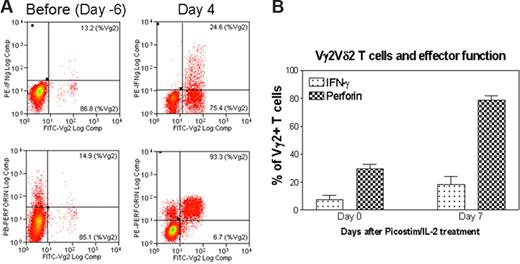
The formation of highly engaged, sustained nanoclusters of Ag-specific Vγ2Vδ2 TCR during the ongoing T-cell activation–expansion raised an immunologic question: do clonally expanded Vγ2Vδ2 T cells bearing the highly engaged TCR nanoclusters or nanodomains gain the functional advantage or ability to sensitize antigens and exert effector function? To address this question, blood Vγ2Vδ2 T cells bearing the high-density TCR nanoclusters were restimulated with phosphoantigen HMBPP and assessed for the ability to rerecognize the antigen and mediate effector function. Surprisingly, a majority of Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were able to rerecognize the phosphoantigen HMBPP and produce cytokines (Figure 7). Whereas on day 4 after the HMBPP/IL-2 treatment, all clonally expanded Vγ2Vδ2 T cells formed TCR nanoclusters and nanodomains with very few, approximately 50-nm TCR dots detectable on cell surface (Figures 5A,B, 6,S2,S3), these cells were able to produce higher levels of antimicrobial cytokine IFN-γ or cytotoxic granule perforin compared with baseline Vγ2Vδ2 T cells that displayed few or no nanoclusters. Similarly, on day 7 after the Picostim/IL-2 treatment, clonally expanded Vγ2Vδ2 T cells bearing highly engaged TCR nanoclusters were able to produce higher levels of IFN-γ or perforin after in vitro stimulation with HMBPP (Figures 4A,B,6,S2,S3).
Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were still capable to rerecognize phosphoantigen and to exert effector function during the phosphoantigen-mediated clonal expansion. (A) Flow cytometry histogram indicating that expanded Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were able to rerecognize phosphoantigen and exert better effector function of IFNγ or perferin production compared with the nonstimulating Vγ2Vδ2 T cells that had few or no nanoclusters. Shown are the representative data from one of 3 monkeys on day 4 after HMBPP/IL-2 treatment. The cells shown in the histograms were CD3-gated (P < .05 for the difference in perforin or IFNγ levels between day 4 and pretested cells). Negative controls using medium alone or an irrelevant peptide did not induce detectable numbers of Vγ2Vδ2 T effector cells. (B) The percentage of Vγ2 T cells that expressed either IFNγ or perforin in response to phosphoantigen HMBPP. Data were derived from PBLs collected from 2 monkeys on day 7 after phosphoantigen Picostim/IL-2 treatment. Error bars represent SEM.
Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were still capable to rerecognize phosphoantigen and to exert effector function during the phosphoantigen-mediated clonal expansion. (A) Flow cytometry histogram indicating that expanded Vγ2Vδ2 T cells bearing high-density TCR nanoclusters were able to rerecognize phosphoantigen and exert better effector function of IFNγ or perferin production compared with the nonstimulating Vγ2Vδ2 T cells that had few or no nanoclusters. Shown are the representative data from one of 3 monkeys on day 4 after HMBPP/IL-2 treatment. The cells shown in the histograms were CD3-gated (P < .05 for the difference in perforin or IFNγ levels between day 4 and pretested cells). Negative controls using medium alone or an irrelevant peptide did not induce detectable numbers of Vγ2Vδ2 T effector cells. (B) The percentage of Vγ2 T cells that expressed either IFNγ or perforin in response to phosphoantigen HMBPP. Data were derived from PBLs collected from 2 monkeys on day 7 after phosphoantigen Picostim/IL-2 treatment. Error bars represent SEM.
Discussion
To our knowledge, the NSOM- and QD-based imaging system generated the best optical resolution (∼50 nm) for an immunofluorescence imaging of individual functional TCR on the cell surface.8,9,12-15,35,36 However, it should be pointed out that, although another type of NSOM, apertureless NSOM (ANSOM) or NSOM with pointed probes,10,11 has overcome the limitation of aperture probes of aperture NSOM and reached a great spatial resolution,37,38 ANSOM has not been successful for such nanoscale resolution imaging of cell membrane proteins because of ANSOM's high-background signal and photobleaching of dyes caused by the metal tip of ANSOM.11,38 Because all commercially available NSOM instruments use the aperture NSOM technique, the combined aperture NSOM and QD may provide a powerful tool for high-resolution imaging of TCR and other membrane proteins.
Whereas the NSOM/QD nanotechnology conferred the best optical resolution detection of approximately 50-nm fluorescence TCR dots, the current study could not provide direct evidence indicating that these approximately 50-nm TCR dots may represent a single individual TCR. Because of the approximate 50-nm limit of a NSOM probe, approximately 20-nm conjugated QDs were detected or displayed as approximately 50-nm dots under NSOM. Although our fluorescence intensity analyses of the conjugated QD on glass substrate and γδ T-cell membrane suggested that the 20 fluorescence counts of individual QDs were the basic fluorescence intensity units and corresponded to approximately 50-nm TCR dots, these approximately 50-nm QD-bound TCR dots might represent 2 TCR molecules. There was always a chance that a small fraction of QDs might bind to TCR in 1:2 binding pattern (one Ab binding to 2 TCR), although we used saturating concentrations of antibodies and conjugated QD to maximize predominant 1:1 binding mode.17 Despite this uncertainty, however, our NSOM/QD-based nanoscale studies focusing on dynamic changes in TCR sizes, and fluorescence intensity allowed us to demonstrate that basic approximately 50-nm TCR dots were able to undergo redistribution and to form TCR nanoclusters, nanodomains, or microdomain during the in vivo clonal expansion of Vγ2Vδ2 T cells.
NSOM images suggest that γδ and αβ TCR are differently distributed on cell surface. The detectable TCR dots on the upper half of a γδ T cell (the lower half contacts with glass substrate) are 4843 plus or minus 1190 (mean values derived from > 7 cells), with approximately 50-nm small dots being dominated. In contrast, fewer but larger TCR dots were predominantly distributed on αβ T cells (1629 ± 1146/per one-half cell). The reasons for different TCR distribution or expression patterns between γδ T cells and αβ T cells are currently not known. Crystallography data suggest that γδ TCR proteins are structurally distinct from αβ TCR. These features may help explain why functional γδ and αβ TCR are distributed differently on cell surface for surveillances or recognizing of their own specific antigens. It is also possible that the difference may be simply due to a difference in expression of CD3 components between γδ and αβ T cells because there is an absence of CD3 δ in TCR complexes of γδ T cells.39,40 The numbers of TCR dots directly observed by NSOM/QD technology were smaller than those wide-ranging numbers of molecules reported by flow cytometry–based indirect calculation.41,42 Smaller numbers of NSOM TCR dots might be attributed to the conjugated QD size (∼20 nm), which limits the capacity of individual QD to readily bind to closely distributed TCR molecules.
Sustained occurrence of TCR nanoclusters in the clonally expanded Vγ2Vδ2 T cells suggested that high-density TCR nanostructures could continuously be assembled on the membrane during the ongoing clonal expansion of daughter T cells after the phosphoantigen/IL-2 stimulation. Because the Vγ2Vδ2 TCR recognizes phosphoantigens, including HMBPP,33,34 it is unlikely that the TCR nanoclusters are formed spontaneously or induced by other cell-surface molecules interacting with their own ligands in the clonally expanded cells after the HMBPP/IL-2 treatment. The nanodomains or microdomains derived from approximately 50-nm TCR or TCR nanoclusters may be somehow similar to the TCR components in the supramolecular activation clusters as seen early in activation of αβ T cells, although they may have different functional aspects.1-4 It is important to note that phosphoantigens, such as Picostim, can be rapidly cleared off from the circulation, with less than 3-minute half-life after intravenous or intramuscular injection43 (personal communication, E. Oldfield, February 2006). The high-density, sustained TCR nanoclusters on the membrane might be required for the daughter Vγ2Vδ2 T cells to sustain or maintain clonal expansion without further phosphoantigen stimulation. This scenario is supported by the recent observation that after a single, brief antigen exposure, rodent αβ CD4 and CD8 T cells can undergo autonomous proliferation and expansion for days without a requirement for further antigen stimulation.44-47 However, it is also possible that the prolonged presence of high-density TCR nanoclusters might be the outcome or effect resulting from continuous clonal expansion. The immune development of these TCR clusters by clonally expanded Vγ2Vδ2 T cells may occur as a result of formation of lipid rafts (membrane rafts) or other membrane-associated lipids during the clonal T-cell expansion.1-4 Alternatively, the immune development of TCR nanoclusters may be driven by turning on TCR-associated membrane protein(s) expression in clonally expanded Vγ2Vδ2 T cells.
The finding that all clonally expanded Vγ2Vδ2 T cells on days 4 and 7 after the phosphoantigen/IL-2 treatment are shown to bear high-density TCR nanoclusters and nanodomains on cell surface suggests that these TCR nanostructures may play a role in antigen recognition or cytokine production. Indeed, these expanded Vγ2Vδ2 T cells bearing TCR nanoclusters or nanodomains on day 4 after phosphoantigen/IL-2 treatment were able to rerecognize phosphoantigen and exert better effector function of cytokine production. TCR nanoclusters or nanodomains may increase the receptor avidity for sensing phosphoantigen as those cross-linked αβ TCRs that possess higher avidity for peptide–MHC complexes.48 Our results imply that the high-density TCR nanoclusters may confer Vγ2Vδ2 T cells the ability to exert helper (IFN-γ) and cytotoxic (perforin) effector functions. Such TCR nanostructures appeared to make Vγ2Vδ2 T cells more readily for exerting antimicrobial effector function in response to the phosphoantigen. Thus, the presence of high-density TCR nanoclusters may arm Vγ2Vδ2 T cells for mounting both innate and adaptive immune responses during infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hassan Jomaa for providing HMBPP, Dr Helen Sicard and Ms Caterine Laplace in Innate-Pharma, Marseille, France for providing Picostim for in vivo activation/expansion of Vγ2Vδ2 T cells, Dr Chris Ye at University of Toronto, Toronto, ON, Canada, for helpful discussion about nanoscale operation, Dr Meilin Chen for helpful suggestion on confocal microscopy, Quantum dot company (currently Invitrogen) for providing Qdot-related technical information, Veeco Company for NSOM technical support, Sherry S. Chen and Qian Feng for assistance with image processing, and Gucheng Zeng and Dr Liyun Zhong for discussion about the optical issues and assistance with image processing.
This work was supported by National Institutes of Health RO1 grants HL64560 (Z.W.C.) and RR13601 (Z.W.C.) and Sino-America Joint grant 30540420311 (J.C.).
National Institutes of Health
Authorship
Contribution: Y.C. designed and performed the research, analyzed and interpreted data, and wrote the paper; L.S. and Z.A. performed research and analyzed data; J.C. provided technological assistance and analyzed data; Z.W.C designed the research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zheng W. Chen, Department of Microbiology and Immunology, Center for Primate Biomedical Research, University of Illinois College of Medicine, Chicago, IL 60612; e-mail: zchen@uic.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal