Abstract
Natural killer (NK) cells have been thought to develop from committed progenitors in the bone marrow. However, a novel pathway of thymus-dependent NK-cell development that produces a unique subset of NK cells expressing CD127 has recently been reported. We now have identified 2 populations of NK progenitors, one in the thymus and the other in the lymph node (LN). Immature double-negative 2 (CD4−CD8−CD44+CD25+) thymocytes have potential to produce NK cells with rearranged T-cell receptor γ genes (Tcrγ+) in vitro. Tcrγ+ NK cells are rare in spleen but relatively abundant in the thymus and LN. Approximately 20% of LN NK cells are Tcrγ+, and they are found at similar levels in both CD127+ and CD127− subsets. Moreover, a subpopulation of LN cells resembling immature thymocytes differentiates into Tcrγ+ NK cells in vitro and also repopulates the NK compartment in lymphopenic mice. Athymic mice lack the LN NK progenitors expressing CD127 as well as Tcrγ+ NK cells. These results suggest that Tcrγ+ NK cells may be generated from unique progenitors in the thymus as well as in the LN.
Introduction
Natural killer (NK) cells are lymphocytes that belong to the innate immune system. They have a large granular morphology and mediate cellular cytotoxicity as well as release chemokines and inflammatory cytokines. They are involved in combating tumors, viral infections, parasites, and bacteria. NK cells develop from hematopoietic stem cells in the bone marrow (BM). Several oligopotent BM precursors that have potential for NK, B, and T cells have been described.1 A committed NK- cell precursor (NKP) has also been identified in the BM.2 NKPs are Lin−CD122+, lack mature NK-cell markers, and do not have NK-cell functions. They progress through a series of developmental intermediates and acquire the full repertoire of receptors as well as cytotoxicity and cytokine production functions.3 In addition to those NK progenitors in the BM, bipotent T/NK-cell precursors are found in the fetal liver, spleen, and blood.4,5 In adult mice, subsets of immature double-negative (CD4−CD8−: DN) thymocytes, termed DN1 (CD44+CD25−) and DN2 (CD44+CD25+), have NK-cell potential.6,7
The rearrangement of T-cell receptor (TCR) genes begins at the DN2 stage.8,9 Specifically, both Tcrγ and Tcrδ gene rearrangements are prominent at the DN2 stage, whereas Tcrβ gene rearrangements are almost absent and increase at the DN3 stage of T-cell development. At the DN3 stage, cells become irreversibly committed to the T-cell lineage, and further development becomes TCR-dependent. Although NK cell potential of thymic T-cell progenitors is well documented, it was assumed that this potential is not followed in steady-state NK-cell development in adult mice, because athymic mice have normal NK cells. However, these studies focused on splenic NK cells, whereas thymus and LN NK cells were not examined.
A subset of NK cells develop in the thymus rather than the BM. We first became aware of the possibility of an NK-cell developmental pathway in the thymus when we discovered T-cell receptor (TCR) γ gene expression during global microarray analysis of NK cells. Through genomic PCR designed to detect TCR gene rearrangements, we showed that NK cells rearrange and express mRNA for Tcrγ but not for Tcrβ or Tcrα. Germ line Tcrδ mRNA expression and low levels of genomic Tcrδ rearrangement were also detected. Because Tcr gene rearrangement occurs during the early stages of T-cell development in the thymus, when NK-cell potential is still present, we hypothesized that NK cells with Tcrγ gene rearrangement developed in the thymus. Expression of TCR has recently been detected in neutrophils,10 indicating that TCR expression is not as stringently regulated as previously thought. However, Tcrγ gene rearrangement detected in NK cells is unlikely to be due to an aberrant event. Indeed, Tcrγ rearrangement proved to be a unique marker of NK cells derived from the thymus. More than half of thymus NK cells in adult mice have rearranged Tcrγ genes, whereas less than 5% of BM and approximately 1% of splenic NK cells do. These NK cells with Tcrγ gene rearrangements (herein termed Tcrγ+ NK cells) are undetectable in athymic Foxn1−/− (nude) mice, indicating that they are thymus-derived.11 Vosshenrich et al12 have also shown that a unique subset of NK cells expressing CD127 (IL-7Rα), and the transcription factor GATA-3 originates in the thymus. A high percentage of thymus NK cells are CD127+, whereas only a small proportion of BM, spleen, and liver NK cells express CD127. CD127+ NK cells are absent in nude mice as well, which confirms their thymic origin. Furthermore, CD127+ thymus-derived NK cells produce high amounts of cytokines, but they are less cytotoxic than conventional NK cells. Thus, thymus-derived NK cells have a distinct phenotype and function. Thymus pathway of NK-cell development also differs from the BM pathway in the transcription factor Id2. In Id2-deficient mice, BM-derived NK cells are almost completely absent, whereas CD127+ NK cells are found in spleen and thymus.13 Other than these studies, little is known about the thymus-dependent pathway of NK-cell development, including the identity of progenitors and whether this pathway produces NK cells in other tissues as well.
We now have used Tcrγ gene rearrangement as a marker for the thymus-dependent NK cells in various tissues and also identified the progenitors that give rise to Tcrγ+ NK cells. Our results show that Tcrγ+ NK cells are relatively abundant in the LN and immature DN thymocytes as well as LN cells resembling DN thymocytes have the potential to produce Tcrγ+ NK cells.
Methods
Mice
C57BL/6 (B6) mice were bred in our animal facility. Nude (Foxn1−/−) B6, Tcrβ/Tcrδ-double knockout B6 (B6.129P2-Tcrbtm1MomTcrdtm1Mom/J), and NOD/SCID/IL-2Rγ−/− mice were from The Jackson Laboratories (Bar Harbor, ME). IL-15−/− mice were from Taconic Farms (Germantown, NY).
Antibodies, fluorescence-activated cell sorting, and analysis
Phycoerythrin (PE)–conjugated anti-NK1.1, CD11b, CD3, CD25, fluorescein isothiocyanate (FITC)–conjugated anti-CD3ε, NK1.1, TCRβ, Tcrγδ, CD19, NKG2A/C/E, NKG2D, Ly49D, Ly49G, Ly49C/I, Ly49H, Mac-1, B220, CD45.2, allophycocyanin (APC)–conjugated anti-mouse NK1.1, CD3, CD25, interferon γ (IFNγ), biotin anti-mouse γδ TCR, CD19, CD3, Ter119, Gr-1, NK1.1, NKG2A/C/E, CD94, CD44, Ly49G, Ly49C/I, Ly49A, interleukin 7 receptor α (IL-7Rα), NKG2D, PerCP-cy5.5–labeled CD3, APC-, PE-, and FITC-conjugated streptavidin were purchased from BD Biosciences (Mississauga, ON). FITC- or PerCP-cy5.5–conjugated anti-CD8 was from Boehringer Mannheim (Indianapolis, IN). Biotinylated anti-CD11b, anti-B220, and FITC-anti Ly5.1 made in-house. Cells were stained with antibodies and sorted as described previously.11
Tcrγ gene rearrangement
Semiquantitative genomic polymerase chain reaction (PCR) to measure the percentages of cells with rearranged Tcrγ gene was carried out as described previously.11
Primary lymphocyte preparation
Cell suspensions were prepared from spleen, thymus, or LN tissue as described.11 For lung and liver, the tissues were perfused with phosphate-buffered saline (PBS), cut into small pieces, and digested with 25 to 50 U/mL DNase I and 250 U/mL collagenase IV. Tissues were then passed through a 70-μm filter and washed. Lymphocytes were isolated by Percoll density (40% and 70% for liver and 44% and 67% for lung; GE Healthcare, Little Chalfont, United Kingdom) centrifugation at 2100 rpm for 20 minutes. Cells in the interface were collected and washed, and red blood cells were lysed.
Isolation of NK progenitors and NK differentiation culture
Thymocytes and LN cells were incubated with 2.4G2 to block the Fc receptor and then stained with FITC-conjugated lineage marker monoclonal antibodies (mAbs; CD3, CD8, TCRβ, TCRγδ, CD19, B220, Mac-1, GR-1, NK1.1, NKG2A/C/E, Ly49G, Ly49D, and Ter119). Lin− cells were pre-enriched by removing Lin+ cells with EasySep FITC Positive Selection kit (Stem Cell Technologies, Vancouver, BC). Cells were then stained with CD44 and CD25 mAbs and sorted into subsets. For BM NKP cells, Lin− cells were stained for NK1.1 and CD122, and NKP (Lin−NK1.1−CD122+) cells were sorted. For NK-cell differentiation, cells were seeded onto preformed OP9 stroma at 2 to 4 × 104 cells in 500 μL minimal essential medium (MEM) with 10% fetal bovine serum (FBS), 150 μmol/L monothioglycerol, 30 ng/mL stem cell factor (SCF), 100 ng/mL recombinant human Fms-like tyrosine kinase 3 ligand (Flt3L), 1 ng/mL IL-7, and 25 ng/mL IL-15. Half of the media was replaced on day 4; if necessary, cells were transferred to new OP9 with new media at a later stage. Cultures were grown for 10 to 12 days. OP9 stroma cells were cultured in α-MEM with 10% FBS. For limiting dilution cultures, cells were directly sorted into 96-well plates and cultured as described for NK differentiation culture in this subsection.
IFNγ production assay
Bulk thymocytes and splenocytes (2 × 106) were cultured in 2 mL of RPMI 1640 medium with 10% FBS, penicillin/streptomycin, 2-mercaptoethanol, 1 ng of IL-12, and 0.5 ng of IL-18 at 37°C for 24 hours. Intracellular staining of IFNγ was performed with the Cytofix/Cytoperm Plus kit (BD Biosciences) according to the manufacturer's protocols and analyzed by fluorescence-activated cell sorting (FACS).
Cytotoxicity assay
MHC-class I deficient mouse lymphoma cell line (RMA-S) cells were labeled with Vybrant carboxyfluorescein diacetate succinimidyl ester (CFDA SE) Cell Tracer kit (Invitrogen, Carlsbad, CA). CFDA SE-labeled RMA-S cells (104) were mixed with either IL-2–activated NK cells at various ratios in 500 μL RPMI 1640 medium + 5% FBS. After 4 hours, the cells were washed and stained with 5 μg/mL propidium iodide and analyzed by FACS. The level of cytotoxicity was determined as the percentage of CFSE+ target cells stained with propidium iodide minus the background level.
Transplantation assays
DN1 and pre-DN2 cells were sorted from IL-15−/− C57BL/6 mice as described in “Isolation of NK progenitors and NK differentiation culture.” Cells (2 × 104) were injected intraperitoneally in 500 μL of PBS into 9 Nod/Scid/IL-2Rγ−/− mice. Three weeks later, blood, spleens, thymuses, BM, and LN were removed and examined by FACS analysis. Lineage marker-negative CD122+ BM cells were isolated and used as positive control for NK progenitors in the transplantation assays. For the thymocyte transplantation experiment, bulk thymocytes from Tcrβ/Tcrδ-double knockout mice were labeled with 1.5 μmol/L CFSE using the Invitrogen Vybrant CFDA SE Cell Tracer kit, and 4 × 106 labeled cells in 500 μL PBS were injected via tail vein into nude mice. After 24 hours, spleen, LN, and peripheral blood were stained with Lin antibody cocktail, anti-CD25, and anti-CD44 and analyzed by flow cytometry.
Results
Thymus NK cells had immature phenotypes but were functionally mature
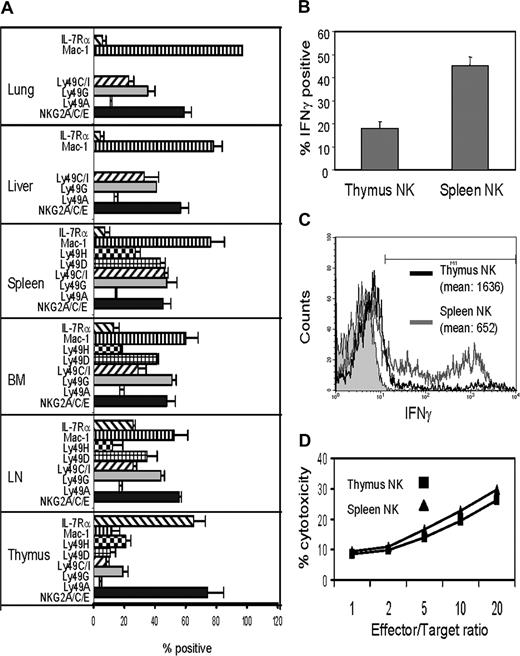
Flow cytometric analysis of NK cells from the thymus, spleen, liver, lung, BM, and lymph nodes showed that thymus NK cells significantly differed from NK cells in other tissues in receptor expression patterns (Figure 1A). Thymus NK were mostly NKG2/CD94hiLy49loMac-1lo, which is characteristic of an immature NK cell phenotype. The percentage of CD127+ cells was very high in thymus NK cells, and LN NK cells were the only other population with a moderate percentage of CD127+ cells. To test the functional maturity of thymus NK cells, they were stimulated in vitro with IL-12 plus IL-18, and IFNγ production was examined by intracellular staining. Approximately 18% of thymus NK cells produced IFNγ whereas 45% of splenic NK cells did (Figure 1B). Although the percentage of IFNγ-producing NK cells in the thymus was significantly smaller than that in the spleen, the amount of IFNγ, as determined by the fluorescence intensity in the IFNγ-positive NK cells from the thymus, was significantly higher than that of spleen NK cells (Figure 1C). Therefore, some thymus NK cells were potent IFNγ producers. To test cytotoxicity of thymic NK cells, thymocytes were cultured with IL-2 and NK cells were purified from the cultures. IL-2–activated thymic NK cells killed RMA-S cells to similar level to that of splenic NK cells (Figure 1D). These results indicated that thymic NK cells were functional despite their immature phenotype.
Thymic NK cells have immature phenotype but are functionally mature. (A) Lymphocytes from various tissues of B6 mice were stained for CD3, NK1.1, and indicated NK-cell receptors and analyzed by multicolor flow cytometry. NK cells were gated by CD3−NK1.1+, and the percentages of NK cells expressing indicated NK receptors over control staining with isotype matched antibodies were determined. Dead cells were gated out by staining with propidium iodide. The results are average (± SD) of more than 3 independent experiments. (B) Thymocytes and spleen cells from Tcrβ−/−δ−/− mice were stimulated in vitro with IL-12 and IL-18 for 24 hours, stained for CD3−NK1.1, fixed and stained for intracellular IFNγ. The percentages of NK cells positively stained for IFNγ over isotype-matched control antibody staining were determined by flow cytometry. The results are averages of 3 independent experiments. (C) Thymocytes and spleen cells were stimulated with IL-12 and IL-18 as above, and the level of intracellular IFNγ in NK cells was determined by flow cytometry. Histograms of control staining with isotype matched control Ab (filled in gray), intracellular IFNγ staining of thymic NK cell (black), and that of spleen NK cells (gray) is shown. The mean fluorescence intensities of positively stained cells are also shown. The results are representative of 3 independent experiments. (D) Thymus and spleen NK cells were stimulated in vitro with IL-2, and killing of RMA-S cells was analyzed. The results are the average of quadruplicate experiments.
Thymic NK cells have immature phenotype but are functionally mature. (A) Lymphocytes from various tissues of B6 mice were stained for CD3, NK1.1, and indicated NK-cell receptors and analyzed by multicolor flow cytometry. NK cells were gated by CD3−NK1.1+, and the percentages of NK cells expressing indicated NK receptors over control staining with isotype matched antibodies were determined. Dead cells were gated out by staining with propidium iodide. The results are average (± SD) of more than 3 independent experiments. (B) Thymocytes and spleen cells from Tcrβ−/−δ−/− mice were stimulated in vitro with IL-12 and IL-18 for 24 hours, stained for CD3−NK1.1, fixed and stained for intracellular IFNγ. The percentages of NK cells positively stained for IFNγ over isotype-matched control antibody staining were determined by flow cytometry. The results are averages of 3 independent experiments. (C) Thymocytes and spleen cells were stimulated with IL-12 and IL-18 as above, and the level of intracellular IFNγ in NK cells was determined by flow cytometry. Histograms of control staining with isotype matched control Ab (filled in gray), intracellular IFNγ staining of thymic NK cell (black), and that of spleen NK cells (gray) is shown. The mean fluorescence intensities of positively stained cells are also shown. The results are representative of 3 independent experiments. (D) Thymus and spleen NK cells were stimulated in vitro with IL-2, and killing of RMA-S cells was analyzed. The results are the average of quadruplicate experiments.
DN2 thymocytes differentiated into Tcrγ+ NK cells
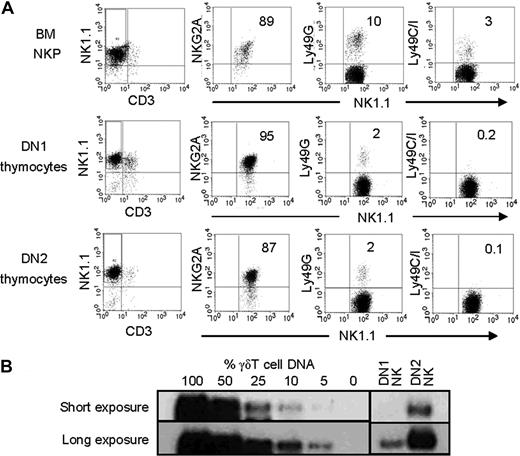
Our previous studies have shown that almost 50% of thymic NK cells are Tcrγ+.11 To identify thymic progenitors that give rise to Tcrγ+ NK cells, we purified DN1 (Lin−CD44+CD25−) and DN2 (Lin−CD44+CD25+) thymocytes and cultured them on OP9 stroma with a cytokine cocktail of IL-15, IL-7, SCF, and Flt3L. BM NKP (Lin−NK1.1−CD122+) cells were used as control. At the end of all cultures, most cells (more than 90% in all cases) were NK1.1+CD3− and expressed CD94/NKG2, whereas minor fractions expressed Ly49 (Figure 2A). NK cells generated from DN1 and DN2 thymocytes in vitro were sorted, and Tcrγ gene rearrangements were examined. Semiquantitative genomic PCR showed that 25%-50% of DN2-derived NK cells and only approximately 5% of DN1-derived NK cells had Tcrγ gene rearrangements (Figure 2B). Because TCR gene rearrangement begins at the DN2 cell stage,8 the results suggest that Tcrγ gene rearrangement takes place in the progenitors, not during differentiation from immature thymocytes into NK cells. Because DN2 thymocytes have much greater potential to produce Tcrγ+ NK cells than DN1 cells, most thymic Tcrγ+ NK cells probably arise from DN2 cells. Thus, the NK-cell potential in fetal and adult DN progenitors, which has been clearly demonstrated previously by other groups,4-7 is realized during NK- cell development.
DN2 thymocytes differentiate in vitro into TCRγ+ NK cells. (A) BM NKPs (top row), thymic DN1 (middle row), and DN2 progenitors (bottom row) were purified and cultured with OP9 stroma and cytokines for differentiation into NK cells. Cells were harvested after 2 weeks and analyzed by flow cytometry for the presence of NK cells (NK1.1+CD3−) as well as expression of indicated receptors on NK cells. Data are representative of 5 independent experiments. (B) NK cells generated in cultures from DN1 and DN2 thymocytes above were purified, and the percentages of Tcrγ+ NK cells were estimated by semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene using γδT cell DNA and fibroblast DNA mixed at set ratios as controls. The total amount of DNA was kept consistent for the PCR. The PCR products were analyzed by Southern blot using Jγ1 probe. Short and long exposures of the same membrane are shown. Images from different parts of the same membrane are divided by lines. The results are representative of 3 independent experiments.
DN2 thymocytes differentiate in vitro into TCRγ+ NK cells. (A) BM NKPs (top row), thymic DN1 (middle row), and DN2 progenitors (bottom row) were purified and cultured with OP9 stroma and cytokines for differentiation into NK cells. Cells were harvested after 2 weeks and analyzed by flow cytometry for the presence of NK cells (NK1.1+CD3−) as well as expression of indicated receptors on NK cells. Data are representative of 5 independent experiments. (B) NK cells generated in cultures from DN1 and DN2 thymocytes above were purified, and the percentages of Tcrγ+ NK cells were estimated by semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene using γδT cell DNA and fibroblast DNA mixed at set ratios as controls. The total amount of DNA was kept consistent for the PCR. The PCR products were analyzed by Southern blot using Jγ1 probe. Short and long exposures of the same membrane are shown. Images from different parts of the same membrane are divided by lines. The results are representative of 3 independent experiments.
A subset of LN NK cells were Tcrγ+ and thymus-dependent
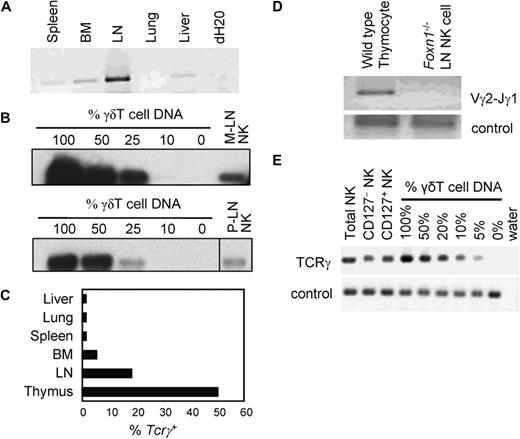
To find out whether Tcrγ+ thymic NK cells exist in other tissues, we purified NK cells from various tissues and tested for Tcrγ gene rearrangement because this is a unique marker of thymus-derived NK cells. NK cells from lung, liver, spleen, and BM have very low levels of Tcrγ gene rearrangement, whereas LN NK cells consistently showed significant levels of Tcrγ gene rearrangement (Figure 3A). Semiquantitative genomic PCR showed that approximately 20% to 25% of both peripheral and mesenteric LN NK cells were Tcrγ+ (Figure 3B,C). To confirm that the LN Tcrγ+ NK cells were thymus-dependent, LN NK cells from nude mice were examined. Tcrγ+ NK cells were undetectable in nude mouse LN, indicating that they are thymus-dependent (Figure 3D). Because Vosshenrich et al have recently reported that thymus-derived NK cells express CD127,12 we purified CD127+ and CD127−LN NK cells and tested Tcrγ gene rearrangement. Unexpectedly, similar levels (∼20%) of Tcrγ gene rearrangement were detected in both NK subsets (Figure 3E).
LN contains thymus-dependent Tcrγ+ NK cells. (A) DNA was extracted from NK cells purified from various tissues of B6 mice. Equivalent amount of NK-cell DNA from each tissue was subjected to genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene and separated by agarose gel electrophoresis. Ethidium bromide staining of the agarose gel is shown. (B) Mesenteric (M-LN) and peripheral (P-LN) LN NK cells were purified by cell sorting and DNA extracted. Semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) was performed and analyzed by Southern blot as in Figure 2B. Images from different parts of the same membrane are divided by lines. (C) NK cells from indicated tissues were purified and subjected to semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene as in panel B, and the approximate percentages of Tcrγ+ NK cells were determined. The results are average of 2 experiments. (D) DNA was extracted from NK cells purified from nude (Foxn1−/−) mouse LN and subjected to genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene as in panel A. Thymocytes from wild-type B6 mice were used as positive control. Genomic PCR for Nkg2a was used as control. Ethidium bromide staining of agarose gel is shown. (E) DNA was extracted from sorted CD127+, CD127−, and bulk LN NK cells from Tcrβ/Tcrδ-double knockout mice. Semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene was performed as in Figure 2B. SYBR Safe (Invitrogen) was used to visualize DNA.
LN contains thymus-dependent Tcrγ+ NK cells. (A) DNA was extracted from NK cells purified from various tissues of B6 mice. Equivalent amount of NK-cell DNA from each tissue was subjected to genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene and separated by agarose gel electrophoresis. Ethidium bromide staining of the agarose gel is shown. (B) Mesenteric (M-LN) and peripheral (P-LN) LN NK cells were purified by cell sorting and DNA extracted. Semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) was performed and analyzed by Southern blot as in Figure 2B. Images from different parts of the same membrane are divided by lines. (C) NK cells from indicated tissues were purified and subjected to semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene as in panel B, and the approximate percentages of Tcrγ+ NK cells were determined. The results are average of 2 experiments. (D) DNA was extracted from NK cells purified from nude (Foxn1−/−) mouse LN and subjected to genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene as in panel A. Thymocytes from wild-type B6 mice were used as positive control. Genomic PCR for Nkg2a was used as control. Ethidium bromide staining of agarose gel is shown. (E) DNA was extracted from sorted CD127+, CD127−, and bulk LN NK cells from Tcrβ/Tcrδ-double knockout mice. Semiquantitative genomic PCR for rearranged Tcrγ (Vγ2-Jγ1) gene was performed as in Figure 2B. SYBR Safe (Invitrogen) was used to visualize DNA.
DN cells in LN differentiated into Tcrγ+ NK cells in vitro
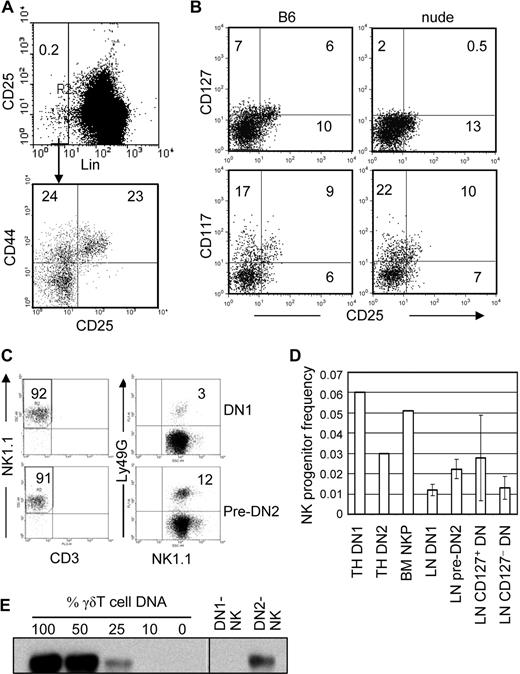
The above finding, that Tcrγ+ NK cells are relatively abundant in the LN but not in spleen, liver, or lung, suggested that they may develop from progenitors in the LN. Minor populations of LN cells that do not express lineage markers (Lin) for mature lymphocytes, monocytes, granulocytes, and erythroid cells and resemble DN thymocytes have been found and termed DN1 (CD44+CD25−) and pre-DN2 (CD44+CD25lo).14 To determine whether they derive from the thymus, we examined the LN of nude mice. Both wild-type and nude mice have comparable numbers of Lin (CD3, CD8, TCRβ, TCRγδ, CD19, B220, Mac-1, NK1.1, NKG2A/C/E, Ly49G, Ly49D)–negative CD44+ cells resembling DN1 or DN2 thymocytes (Figure 4A). However, they differ in the expression of CD127. A significant fraction of DN1 and pre-DN2 cells in wild-type B6 LN expressed CD127, albeit at low levels. In contrast, very few of those in nude mice expressed CD127. Wild type and nude mouse DN1/pre-DN2 did not significantly differ in CD117 (c-kit) expression. These results suggest that the DN population in LN is heterogeneous, and CD127+ DN cells seem to derive from the thymus (Figure 4B).
DN1 and pre-DN2 cells in LN are thymus-dependent NK-cell progenitors. (A) LN cells from B6 mice were stained with Lin antibody cocktail, anti-CD25, and anti-CD44 and analyzed by flow cytometry. Lin− cells were gated, and the percentages of DN1 (CD44+CD25−) and pre-DN2 (CD44+CD25lo) cells were determined. The numbers in the dot plots show the percentage of cells in each region. (B) LN cells from nude (Foxn1−/−) B6, and wild-type B6 mice were analyzed for the presence of IL-7Rα (CD127) and c-Kit (CD117) expression on their DN1 and pre-DN2 populations. Lin−CD44+ LN cells were gated, and CD25 and CD127/CD117 expression was analyzed by flow cytometry. Numbers indicate percentages of positively stained cells. (C) DN1 (top) and pre-DN2 (bottom) LN cells were purified from IL-15–deficient B6 mice and cultured for NK-cell differentiation in vitro. Cells recovered from the cultures were analyzed by flow cytometry for the presence of NK cells (left), and expression of Ly49G on NK cells (right). (D) Limiting numbers of DN1 and DN2 thymocytes, DN1 and pre-DN2 LN cells, and CD127+ and CD127− LN DN cells were sorted by FACS and directly deposited into 96-well plates with preestablished OP9 stroma. The cells were cultured for 3 weeks, as in the bulk cultures above, for NK-cell differentiation, and wells with growing cells were scored. The frequency of NK progenitors in each population was determined by L-Calc software (StemCell Technologies). The results are the averages of 3 independent experiments for LN DN populations and 2 independent experiments for thymus DN1, DN2, and BM NKPs. (E) LN DN1 and pre-DN2 cells from B6 mice were purified and cultured for NK-cell differentiation as in panel C. NK cells at the end of culture were purified and subjected to semiquantitative genomic PCR to determine the frequency of cells with rearranged Tcrγ (Vγ2-Jγ1) as in Figure 2B. Images from different parts of the same membrane are divided by lines.
DN1 and pre-DN2 cells in LN are thymus-dependent NK-cell progenitors. (A) LN cells from B6 mice were stained with Lin antibody cocktail, anti-CD25, and anti-CD44 and analyzed by flow cytometry. Lin− cells were gated, and the percentages of DN1 (CD44+CD25−) and pre-DN2 (CD44+CD25lo) cells were determined. The numbers in the dot plots show the percentage of cells in each region. (B) LN cells from nude (Foxn1−/−) B6, and wild-type B6 mice were analyzed for the presence of IL-7Rα (CD127) and c-Kit (CD117) expression on their DN1 and pre-DN2 populations. Lin−CD44+ LN cells were gated, and CD25 and CD127/CD117 expression was analyzed by flow cytometry. Numbers indicate percentages of positively stained cells. (C) DN1 (top) and pre-DN2 (bottom) LN cells were purified from IL-15–deficient B6 mice and cultured for NK-cell differentiation in vitro. Cells recovered from the cultures were analyzed by flow cytometry for the presence of NK cells (left), and expression of Ly49G on NK cells (right). (D) Limiting numbers of DN1 and DN2 thymocytes, DN1 and pre-DN2 LN cells, and CD127+ and CD127− LN DN cells were sorted by FACS and directly deposited into 96-well plates with preestablished OP9 stroma. The cells were cultured for 3 weeks, as in the bulk cultures above, for NK-cell differentiation, and wells with growing cells were scored. The frequency of NK progenitors in each population was determined by L-Calc software (StemCell Technologies). The results are the averages of 3 independent experiments for LN DN populations and 2 independent experiments for thymus DN1, DN2, and BM NKPs. (E) LN DN1 and pre-DN2 cells from B6 mice were purified and cultured for NK-cell differentiation as in panel C. NK cells at the end of culture were purified and subjected to semiquantitative genomic PCR to determine the frequency of cells with rearranged Tcrγ (Vγ2-Jγ1) as in Figure 2B. Images from different parts of the same membrane are divided by lines.
To test their NK potential, LN DN1 and pre-DN2 cells were purified from IL-15-deficient B6 mice, which have almost no mature NK cells, and then cultured in the same NK-cell differentiation conditions as described for thymus DN progenitors. They differentiated into NK cells very efficiently, the majority of cells at the end of the culture being NK1.1+CD3−, and some expressed Ly49G, indicating that they became mature NK cells (Figure 4C). The same results were also obtained with wild-type B6 mice (data not shown). We further analyzed the frequency of NK progenitors in various cell populations by limiting dilution analyses. Approximately 1 of 16 DN1 and 1 of 33 DN2 cells in the thymus and approximately 1 of 83 DN1 and 1 of 45 pre-DN2 cells in the LN differentiated into NK cells in culture. When LN DN cells were divided by CD127 expression, approximately 1 of 36 CD127+ and 1 of 77 CD127− DN cells differentiated into NK cells (Figure 4D). Approximately 1 of 20 BM NK progenitors (Lin−CD122+) used as positive control differentiated into NK cells in the vitro cultures in this study. Our results with thymus DN1, DN2, and BM NKP are comparable with the previously published results.2,15 Thus, NKPs are found in both thymus-dependent CD127+ and thymus-independent CD127− subsets of LN DN cells.
The LN DN-derived NK cells were purified by cell sorting, and their Tcrγ gene rearrangement status was examined by genomic PCR. As in the DN thymocyte cultures, LN DN1-derived NK cells had negligible levels of Tcrγ gene rearrangement, whereas more than 25% of pre-DN2-derived NK cells had Tcrγ gene rearrangements (Figure 4E). Although LN DN1 cells have NK potential, they do not differentiate into Tcrγ+ NK cells. Thus, approximately 20% of LN NK cells that are Tcrγ+, as shown in Figure 3B, may derive, at least in part, from LN pre-DN2 cells. It is unknown how much LN DN1 progenitors contribute to the NK-cell population in the LN.
LN DN progenitors had NK-cell potential in vivo
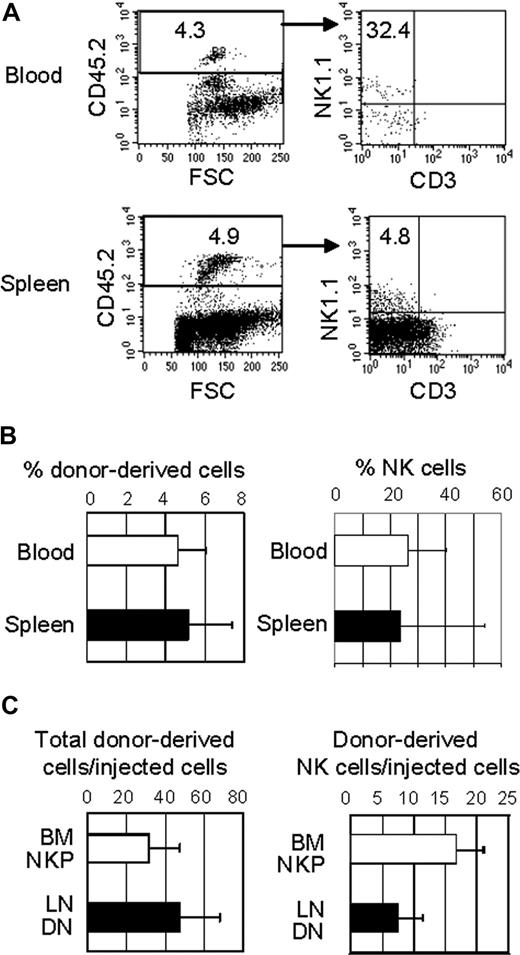
LN DN1 and pre-DN2 progenitors were sorted from IL-15−/− mice and injected intraperitoneally into nonirradiated Nod/SCID/IL-2Rγ−/− mice, which have greatly reduced lymphocytes, including NK cells. After 3 to 4 weeks, tissues were examined for the presence of donor-derived CD3−NK1.1+ cells. Because the donor and the host strains differ in NK1.1 and CD45, donor-derived lymphocytes NK cells could be identified by CD45.2+ and NK1.1+, respectively. Donor-derived NK cells (CD45.2+NK1.1+CD3−) were undetectable in the BM (data not shown) but were detectable in the blood and spleen (Figure 5A). The percentages of donor-derived cells (CD45.2+) were approximately 5% of mononuclear cells in the blood and spleen of the recipient mice (Figure 5B). The percentages of NK cells among the donor-derived cells significantly varied, ranging from approximately 5% to nearly 60% in the spleen and 10% to 36% in the blood. It seems that the progenitors have limited proliferative capacity, and NK cellsderived from the LN progenitors seem to have a short half life. The percentages of the donor-derived cells as well as the percentages of donor-derived NK cells declined rapidly after 4 weeks after transplantation. Nevertheless, these results show that the LN DN populations differentiate into NK cells in vivo. Relative to conventional NK progenitors in the BM, the proliferative capacity of LN DN cells was comparable with that of BM NKP, whereas their NK potential was approximately a half of BM NKP (Figure 5C). Some donor-derived cells expressed CD3 at low levels, but no B cells (CD19+) were detected (data not shown).
LN DN cells develop into NK cells in lymphopenic mice. LN DN1/pre-DN2 cells were purified from IL-15−/− mice and injected intraperitoneally (104 per mouse) into nonirradiated NOD/Scid/IL-2Rγ−/− mice. After 3 weeks, lymphocytes from the recipient mice were analyzed by flow cytometry. (A) Donor-derived lymphocytes in the recipients' blood and spleens were identified by anti-CD45.2 and NK cells among donor-derived lymphocytes were detected. (B) The percentages of donor-derived cells in the recipients' blood and spleen (left) and NK cells among donor-derived lymphocytes (right) were determined by flow cytometry. Similar results were obtained with LN DN cells from normal B6 mice. (C) The proliferative capacity (left) of BM NKP cells and LN DN cells was compared by dividing the number of total donor-derived cells in the recipients' spleens by the number of injected cells. The NK-cell potential (right) of BM NKP cells and LN DN cells was also compared by dividing the number of donor-derived NK cells in the recipients' spleen by the number of injected cells. The donor-derived cells in the BM and blood were not included in the calculations as they were much lower than those in the spleen.
LN DN cells develop into NK cells in lymphopenic mice. LN DN1/pre-DN2 cells were purified from IL-15−/− mice and injected intraperitoneally (104 per mouse) into nonirradiated NOD/Scid/IL-2Rγ−/− mice. After 3 weeks, lymphocytes from the recipient mice were analyzed by flow cytometry. (A) Donor-derived lymphocytes in the recipients' blood and spleens were identified by anti-CD45.2 and NK cells among donor-derived lymphocytes were detected. (B) The percentages of donor-derived cells in the recipients' blood and spleen (left) and NK cells among donor-derived lymphocytes (right) were determined by flow cytometry. Similar results were obtained with LN DN cells from normal B6 mice. (C) The proliferative capacity (left) of BM NKP cells and LN DN cells was compared by dividing the number of total donor-derived cells in the recipients' spleens by the number of injected cells. The NK-cell potential (right) of BM NKP cells and LN DN cells was also compared by dividing the number of donor-derived NK cells in the recipients' spleen by the number of injected cells. The donor-derived cells in the BM and blood were not included in the calculations as they were much lower than those in the spleen.
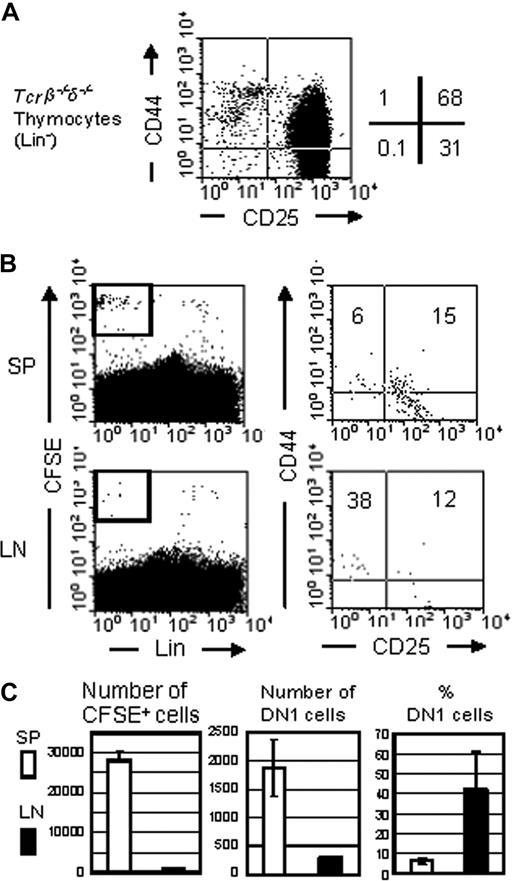
To further test whether some DN cells in the LN derive from the thymus, we isolated thymocytes from Tcrβ/Tcrδ-double knockout mice, labeled them with CFSE, and injected them into nonirradiated nude B6 mice. The donor cells were mostly DN2 and DN3 cells with a small fraction of DN1 cells and no mature thymocytes (Figure 6A). A day after the injection, lymphocytes in the spleen and LN of the recipients were analyzed for the presence of donor cells as well as their phenotype. Only a small fraction of injected cells was recovered in the recipients, mostly in the spleen (Figure 6B), and the ratio of donor DN1 and DN2 in the recipient spleens was similar to that in the donor thymus. In contrast, a high portion (∼40%) of the donor cells found in the recipient LN were DN1 cells, although a much smaller number of donor cells were recovered in the LN (Figure 6C). These results suggest that the migration of DN1 thymocytes into nude mouse LN may be nonrandom, and some DN1 cells injected intravenously seem to have the capacity to cross the high endothelial venule to migrate into LN.
DN1 thymocytes have the capacity to migrate to the LN. (A) Bulk thymocytes from Tcrβ/Tcrδ-double-knockout mice were stained for Lin, anti-CD44, and anti-CD25 and characterized by flow analysis. (B) CFSE labeled bulk thymocytes (4 × 106) from Tcrβ/Tcrδ-double knockout mice were injected intravenously into nude mice. A day after transplantation, spleen and LN cells were stained with Lin antibody cocktail, anti-CD25, and anti-CD44 and analyzed by flow cytometry. CFSE-positive donor-derived cells were gated (left), and the percentages of DN1 (CD44+CD25−), DN2 (CD44+CD25+), and DN3 (CD44−CD25+) cells were determined (right). (C) Total donor-derived cell number seeding the spleen and LN was calculated (right), followed by number and percentage of DN1 cells within this population (middle and left).
DN1 thymocytes have the capacity to migrate to the LN. (A) Bulk thymocytes from Tcrβ/Tcrδ-double-knockout mice were stained for Lin, anti-CD44, and anti-CD25 and characterized by flow analysis. (B) CFSE labeled bulk thymocytes (4 × 106) from Tcrβ/Tcrδ-double knockout mice were injected intravenously into nude mice. A day after transplantation, spleen and LN cells were stained with Lin antibody cocktail, anti-CD25, and anti-CD44 and analyzed by flow cytometry. CFSE-positive donor-derived cells were gated (left), and the percentages of DN1 (CD44+CD25−), DN2 (CD44+CD25+), and DN3 (CD44−CD25+) cells were determined (right). (C) Total donor-derived cell number seeding the spleen and LN was calculated (right), followed by number and percentage of DN1 cells within this population (middle and left).
Discussion
We have previously found a subset of NK cells that has rearranged Tcrγ genes. Tcrγ+ NK cells are thymus-dependent in that they are not detected in athymic mice.11 We now have further characterized Tcrγ+ NK cell development and found that this pathway includes the LN. Almost 20% of LN NK cells are Tcrγ+. Moreover, we have identified progenitors for Tcrγ+ NK cells in the thymus and LN. Although both DN1 and DN2 thymocytes have NK potentials, most Tcrγ+ NK cells in the thymus seem to derive from DN2 thymocytes. NK progenitors resembling DN thymocytes are also found in the LN. Both DN1 (Lin−CD44+CD25−) and pre-DN2 (Lin−CD44+CD25lo) LN cells have NK potential, as demonstrated by their capacities to differentiate into NK cells in vitro as well as in lymphopenic mice upon transplantation. Of those, pre-DN2 cells have higher potential for Tcrγ+ NK cells than DN1 LN cells, suggesting that Tcrγ+ NK cells in LN may develop, at least in part, from pre-DN2 LN cells. However, the proportion of Tcrγ+ NK cells in LN that develop from progenitors in LN versus those that develop in the thymus and emigrate to LN is currently unknown.
Vosshenrich et al12 described CD127+ LN NK cells that are thought to be thymus-derived. We have also shown that Tcrγ+ NK cells in LN are thymus-dependent in that they are absent in nude mice. However, a similar level of Tcrγ gene rearrangement is unexpectedly detected in both CD127+ and CD127− LN NK cells. Thus, the relationship between CD127+ NK cells and Tcrγ+ NK cells in LN is still unclear. It seems that not all thymus-dependent NK cells are Tcrγ+. For example, NK cells that develop from DN1 thymocytes are unlikely to be Tcrγ+. At the same time, some thymus-dependent NK cells (Tcrγ+) seem to be CD127−. There may be at least 2 different populations of thymus-dependent LN NK cells; one develops in the thymus and emigrates to LN and the other develops from thymus-derived progenitors in LN. Alternatively, it is possible that CD127 is only transiently expressed on thymus-derived LN NK cells and it is lost as they further differentiate.
NKPs among LN DN cells are heterogeneous and include both thymus-dependent and -independent NKPs. They also have potentials to generate both Tcrγ+ and Tcrγ− NK cells. Among LN DN cells, CD127+ cells, which have the capacity to differentiate into NK cells in vitro, seem to be thymus-dependent in that they are greatly reduced in nude mice. Our thymocyte transplantation experiments also suggest that some DN1 thymocytes may have the capacity to migrate into LN. Therefore, CD127+ LN DN cells may derive from DN1 thymocytes. This is consistent with the idea that early T-cell progenitors have the capacity to migrate out of the thymus and colonize primary lymphoid organs without losing their differentiation potential. Lambolez et al16 demonstrated that DN2 and DN3 progenitors migrate to the gut and produce CD8αα+ intraepithelial T cells in the gut cryptopatches. On the other hand, LN DN cells are unable to differentiate into T cells in vivo, because the LN environment does not support T-cell development. It is believed that LN DN1 progress to a pre-DN2 stage (CD44+CD25lo), but then further T-cell development is blocked as a result of a lack of expression of wingless-related MMTV integration site-4 (Wnt4).14 Indeed, c-kit (CD117)loCD127+ DN1 LN cells are able to differentiate into mature T cells when cocultured with stroma cells expressing Wnt4. However, the relationship between DN1 thymocytes and LN DN1 is still unclear. DN1 thymocytes are heterogeneous and include CD117−CD127+ and CD117hiCD127−subsets.7 The expression of CD127 is mutually exclusive in thymus progenitors, and T-cell and NK-cell potential is only present in CD117+ DN1 cells. LN DN1 cells are also heterogeneous with respect to CD117 and CD127.14 Our results suggest that CD127+ LN DN cells are thymus-derived. Whereas thymus CD127+ DN1 thymocytes have no NK-cell potential in vitro,7 CD127+ LN DN cells efficiently differentiate into NK cells under the same conditions. Therefore, it appears that thymic CD127−CD117+ DN progenitors possessing NK-cell potential migrate to the LN and up-regulate CD127.
Previous studies have suggested that LN progenitors are thymus-independent.17-19 Guy-Grand et al20 examined LN DN cells in nude mice and showed that a population of CD3−CD4−CD8−CD19− cells were present. However, they did not include NK-cell markers to define the Lin− population and noted that 60% of the DN1 cells had NK-cell markers. Therefore, BM-derived NK cells probably made up the bulk of the DN population of nude mouse LN in their study. Nor did they distinguish between CD127+ and CD127− DN progenitors, of which the CD127+ population is small in wild-type mice; their absence would not be easily detectable in nude mice without staining for CD127+ DN cells directly. In contrast, we included multiple NK-cell markers to exclude mature NK cells in the isolation of LN DN progenitors in our study, and Lin (CD3, CD8, TCRβ, TCRγδ, CD19, B220, Mac-1, NK1.1, NKG2A/C/E, Ly49G, Ly49D)–negative, CD127+ CD44+ CD25−/lo cells are absent in nude mouse LN, indicating their thymus origin.
An LN pathway of NK-cell development has also been demonstrated in humans. There are 2 main subsets of human NK cells21 : CD56dim, which have high cytotoxicity but poor cytokine production, and CD56bright, which have low cytotoxicity but are potent cytokine producers. It is noteworthy that CD56bright NK cells are 10 times more frequent in the LNs than in the blood, and the LNs contain all developmental intermediates spanning from CD34+ progenitors to CD56bright NK cells.22 It is now becoming apparent that different subsets of NK cells exist in the mouse, as in humans.12,23 Until recently, NK cells were divided by their maturational level, and it appeared that all mature NK cells shared the same phenotype and function. Hayakawa et al23 have defined 2 mature NK-cell subpopulations: a Mac-1hiCD27hi and a Mac-1hiCD27lo population. They differ in receptor expression, tissue distribution, proliferation, function, and chemokine sensitivity. Thymus-dependent NK cells now represent another subset of NK cells in the mouse. These NK cells are NKG2/CD94hi, Ly49lo, Mac-1lo. Thymus-dependent NK cells seem to be more efficient cytokine producers. Vosshenrich et al12 showed that CD127+ thymus-derived NK cells produced more IFNγ, granulocyte macrophage–colony-stimulating factor, and tumor necrosis factor than spleen NK cells. We have also found that a lower percentage of thymus NK cells produced IFNγ after stimulation, but those NK cells that do produce IFNγ do so at higher levels than spleen NK cells. In terms of cytotoxic potential, Vosshenrich et al12 showed that freshly isolated thymic NK cells from RAG2-deficient mice show very low cytotoxicity compared with that of spleen NK cells.12 It seems thymic NK cells acquire cytotoxic function upon stimulation with IL-2, though, as shown in our study. It should also be noted that a previous study also demonstrated that fresh NK cells from fetal thymus have normal cytotoxicity.24 It appears that the identity of the progenitors and the environment in which the NK cells develop greatly influences their phenotype and function. The thymus-dependent pathway of NK-cell development differs from the BM pathway in location, precursors, and output.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute of Canada and the Canadian Institute of Health Research. L.L.V. was supported by the Michael Smith Foundation for Health Research.
Authorship
Contribution: L.L.V. and T.Y.F.H. designed and performed the research, analyzed data, and wrote the manuscript, and F.T. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumio Takei, Terry Fox Laboratory, 675 West 10th Ave, Vancouver, British Columbia, Canada V5Z 1L3; e-mail: ftakei@bccrc.ca.
References
Author notes
*L.L.V. and T.Y.F.H. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal