Abstract
Neuropilin-1 (NRP1) and NRP2 are cell surface receptors shared by class 3 semaphorins and vascular endothelial growth factor (VEGF). Ligand interaction with NRPs selects the specific signal transducer, plexins for semaphorins or VEGF receptors for VEGF, and promotes NRP internalization, which effectively shuts down receptor-mediated signaling by a second ligand. Here, we show that the sulfated polysaccharides dextran sulfate and fucoidan, but not others, reduce endothelial cell-surface levels of NRP1, NRP2, and to a lesser extent VEGFR-1 and VEGFR-2, and block the binding and in vitro function of semaphorin3A and VEGF165. Administration of fucoidan to mice reduces VEGF165-induced angiogenesis and tumor neovascularization in vivo. We find that dextran sulfate and fucoidan can bridge the extracellular domain of NRP1 to that of the scavenger receptor expressed by endothelial cells I (SREC-I), and induce NRP1 and SREC-I coordinate internalization and trafficking to the lysosomes. Overexpression of SREC-I in SREC-I–negative cells specifically reduces cell-surface levels of NRP1, indicating that SREC-I mediates NRP1 internalization. These results demonstrate that engineered receptor internalization is an effective strategy for reducing levels and function of cell-surface receptors, and identify certain sulfated polysaccharides as “internalization inducers.”
Introduction
Coordinate development of the vascular system and adult angiogenesis are regulated by endothelial cell-surface receptors activated by soluble mediators,1-3 and by insoluble mediators bound to cells or extracellular matrix.4-6 Neuropilin-1 (NRP1) and NRP2 are endothelial cell receptors shared by members of the class 3 semaphorin and VEGF families,7-11 serving as “hubs” for different ligands. Thus, NRP1 can bind semaphorin3A (Sema3A), Sema3B, Sema3C, heparin-binding forms of VEGF-A, VEGF-B, and placenta growth factor-2 (PlGF2); and NRP2 can bind Sema3B, Sema3C, Sema3F, VEGF-A, VEGF-C, and VEGF-D.10,12 Structurally, NRP1 and NRP2 have an extracellular, a single transmembrane, and a short cytoplasmic domain.10,11 Within the NRP1 extracellular domain, 3 subdomains were identified: the a1/a2 (CUB) domain at the N-terminus with homology to complement components, followed by the b1/b2 (coagulation factor V/VIII) domain and the c (C-terminal MAM) domain.13 Sema3A binds to the a1/a2/b1 domains, whereas VEGF-A binds to the b1/b2 domain.14,15
Previous studies have shown that NRP1 uses the VEGF receptor 2 (VEGFR-2) to transduce VEGF165 signals16 and plexin-A1, plexin-A2, or plexin-A4 to transduce Sema3A signals,17-19 and that NRP1 can orderly mediate signals by these different ligands by complex mechanisms, including internalization.20-22 NRP1-null mice are embryonically lethal, and display severe abnormalities in the nervous and cardiovascular systems.23 NRP2-deficient mice display defective retinal neovascularization in response to ischemia.24 Mice lacking both NRP1 and NRP2 are more severely affected than mice lacking only NRP1.25 In adult mice, monoclonal antibodies against NRP1 inhibit angiogenesis and vascular remodeling.26 These observations point to a critical role of NRP1 and NRP2 in angiogenesis.
The b1/b2 extracellular subdomain of NRP1 has heparin-binding capacity.14,27 Heparin enhances the binding of Sema3A and VEGF165 to NRP1 and augments their biologic functions.9,21,28,29 Many glycosaminoglycans and sulfated polysaccharides can bind to a variety of proteins and lipoproteins in mammalian tissues and modulate their function.30 Heparin can bind to low-density lipoprotein (LDL) and compete for LDL binding to LDL receptors.30 Fucoidan and dextran sulfate, but not heparin, are ligands for acetylated-LDL (Ac-LDL) receptors and can compete for Ac-LDL binding to Ac-LDL receptors.31-34
Cell-surface receptors for Ac-LDL and other chemically modified lipoproteins, defined as scavenger receptors, are uniformly expressed on macrophages31,35 and to a lesser extent on endothelial cells and selected endothelial progenitor cells.36,37 Endothelial cell–associated scavenger receptors include CD36, also identified as scavenger receptor class B type I (SR-BI), lectinlike oxidized LDL receptor-1 (LOX-1), collectin placenta 1 (CL-P1), FEEL-1/Stabilin-1/CLEVER-1, and scavenger receptor expressed by endothelial cells I (SREC-I) and II.38,39 In addition to binding to Ac-LDL and oxidized-LDL, scavenger receptors can bind structurally different ligands, including selected sulfated polysaccharides. Endothelial scavenger receptors have been implicated in the pathogenesis of atherosclerosis, but their roles in angiogenesis are not clear.34,40,41
Taking advantage of endothelial cell expression of both scavenger receptors and NRP1, we show here that certain sulfated polysaccharides mediate the bridging of NRP1 to scavenger receptors, and promote the internalization of cell-surface NRP1 resulting in desensitization of endothelial cells to Sema3A and VEGF165. Thus, we demonstrate that certain sulfated polysaccharides act as “internalization inducers” as they promote internalization and sequestration of critical receptors away from the cell surface where they are active.
Methods
Reagents and cytokines
Porcine heparin sodium salt, chondroitin sulfate A and B, shark cartilage chondroitin 6-sulfate (chondroitin sulfate C), dextran, dextran sulfate Mr 8000, Mr 500 000, fucoidan Mr 66 410, and bovine fibronectin were from Sigma-Aldrich (St Louis, MO). Low-molecular-weight fucoidan was a gift of Dr Didier Letourneur (Inserm, Paris, France). Bovine kidney heparan sulfate was from Calbiochem (San Diego, CA). Recombinant human VEGF165, chimeric rat NRP1/Fc, human Sema3A/Fc, SREC-I/Fc, gp130/Fc, and B7-1/Fc were from R&D Systems (Minneapolis, MN). Ac-LDL and 1,1′-dioctadecyl-Ac-LDL (DiO-Ac-LDL) were from Biomedical Technologies (Stoughton, MA). Alexa 594 Ac-LDL was from Invitrogen (Carlsbad, CA).
Cells and culture conditions
Primary human umbilical vein endothelial cells (HUVECs) were prepared and maintained as previously described.42 HUVECs were used between the second and the fifth passage. The cell line RS4;11 (RS4; ATCC, Manassas, VA) was maintained in RPMI 1640 with 10% fetal bovine serum (FBS); the cell lines HS-5, COS7, and HEK-293 (ATCC) were maintained in DMEM with 10% FBS. The murine plasmacytoma MOPC-315 cell line, a gift of Dr B. Mock (NIH, Bethesda, MD) was propagated in RPMI 1640 with 10% FBS and 55 μM 2-mercaptoethanol.
NRP1-heparin–binding assay
Heparin was biotinylated as described previously.43 Biotinylated heparin (1.8 U/mL in PBS) was injected onto the flow cell of the Sensor Chip SA (Biacore, Piscataway, NJ). Biotinylated heparin (500 resonance units[RU]) was immobilized. NRP1-heparin binding was analyzed using the BIAcore 3000 system (Biacore). NRP1/Fc protein (20 nM) was diluted in HEPES buffer saline containing 0.005% Surfactant P20 (HBS-P; Biacore) injected over the heparin-coated or control flow cell surface at a flow rate 50 μL/min at 25°C. Association and dissociation phases were evaluated for 2 minutes. The sensor chip was regenerated with pulse of 2 M NaCl for 30 seconds. The data were analyzed using BIAevaluation software (Biacore).
Affinity purification with immobilized polysaccharide gels
NRP1/Fc or gp130/Fc was incubated with heparin-gel, dextran sulfate-gel, or maltose-gel (EY Laboratories, San Mateo, CA) at 25°C for 1 hour. After washing with PBS three times, gels were suspended in tricine sodium dodecyl sulfate (SDS) sample buffer (Invitrogen), and incubated at 100°C for 5 minutes. Extracts were separated by SDS–polyacrylamide gel electrophoresis (PAGE), transferred onto nitrocellulose membrane (Invitrogen), blocked with 5% skim milk in PBS 0.1% Tween20, and immunostained using biotin-conjugated anti–human IgG1 Ab (Zymed, South San Francisco, CA), followed by incubation with an HRP-conjugated streptavidin (Zymed). Immunocomplexes were visualized using a chemiluminescence detection system (GE Healthcare, Little Chalfont, United Kingdom).
Flow cytometric analysis
HUVECs were detached with 5 mM EDTA in PBS, washed with 1% FBS buffer (MEDIUM199, 1% FBS, 10 mM HEPES), and incubated with polysaccharides. After rinsing twice with 1 M NaCl with 0.2% BSA followed by 2 rinses with binding buffer, cells were stained with PE anti–BDCA-4 (NRP1) mAb (Miltenyi Biotec, Auburn, CA), PE anti–VEGFR-1 mAb (R&D Systems), PE anti–VEGFR-2 mAb (R&D Systems), FITC anti–CD31 mAb (BD Biosciences, San Jose, CA), PE anti–VE-cadherin mAb (R&D Systems), PE anti-CXCR4 mAb (BD Biosciences, San Jose, CA), or anti-NRP2 mAb (Santa Cruz Biotechnology, Santa Cruz, CA), anti-gp130 mAb (BD Biosciences), or anti–SREC-I Ab (R&D Systems) followed by Alexa 488–conjugated antimouse Ab or Alexa 488–conjugated antigoat Ab (Invitrogen). Data were collected using a FACScalibur cytofluorometer (BD, Franklin Lakes, NJ).
Laser confocal microscopy
Endothelial cells growing onto glass chamber slides (Nalge Nunc International, Rochester, NY) coated with 5 μg/mL fibronectin were incubated in 1% FBS buffer with DS500 (8 μg/mL) and protease inhibitor cocktail III (Calbiochem) at 37°C for the indicated times. The medium was replaced with 4% (wt/vol) paraformaldehyde for fixation, and cells were washed with PBS and permeabilized with 0.1% Triton X100 in PBS. Cells were then stained with mouse antihuman NRP1 mAb, rabbit antihuman Lamp1 Ab (Affinity BioReagents, Golden, CO), or goat antihuman SREC-I Ab in PBS with 3% BSA and 3% FBS at 4°C for 16 hours. After washing with PBS, slides were incubated with Alexa 488–conjugated anti–mouse IgG Ab, Alexa 594–conjugated anti–rabbit IgG Ab, or Alexa 488– or Alexa 594–conjugated anti–goat IgG Ab (Invitrogen), washed, and mounted with VECTASHIELD with DAPI (Vector Laboratories, Burlingame, CA). Images were obtained using a LSM 510 Zeiss confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY).
Western blot analysis
After incubation of endothelial cells in 1% FBS buffer with 2 μg/mL DS500, cell pellets were suspended in lysis buffer (0.5% NP40, 150 mM NaCl, 10 mM TrisHCl, pH 7.4) with protease inhibitor cocktail III. Cell lysates were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and immunostained using rabbit anti-NRP1 Ab (Santa Cruz Biotechnology), followed by incubation with an HRP-conjugated anti–rabbit IgG Ab (GE Healthcare). Immunocomplexes were visualized using a chemiluminescence detection system. The membrane was reblotted with goat antiactin Ab (Santa Cruz Biotechnology). Band intensities were measured by NIH image.
Forced expression of SREC-I and NRP1
Human NRP1 in pCMV6-XL4 was from OriGene Technologies (Rockville, MD). Human SREC-I in pCMV-SPORT6 was from Open Biosystems (Huntsville, AL). Plasmid vectors were transfected into HEK-293 or CHO-K1 cells with the use of Lipofectamine 2000 (Invitrogen). Cells were harvested 2 days after transfection for analysis.
Enzyme-linked immunosorbent assay–based binding assays
Flat-bottom microtiter plates (96 well, Immulon 4HBX; Thermo Labsystems, Franklin, MA) were coated with control human IgG1 (Calbiochem) or SREC-I/Fc chimeric protein (2 μg/mL). After blocking with PBS 0.1% Tween20 5% BSA, NRP1/Fc or control B7–1/Fc in PBS 0.1% Tween20 1% BSA was added with or without polysaccharide. Bound NRP1/Fc or B7-1/Fc was detected by anti-His mAb (Invitrogen), followed by HRP-conjugated anti–mouse IgG Ab (GE Healthcare). Reactions were visualized with tetramethoxybenzene peroxidase substrate (KPL, Gaithersburg, MD) followed by addition of 1 M H2SO4, and read at 450 nm.
Cell-binding assays
HUVECs were incubated in 1% FBS buffer with or without polysaccharides. Cells were washed with 1 M NaCl twice, followed by washing with 1% FBS buffer. Cells were incubated with Sema3A/Fc (2 μg/mL) or biotinylated VEGF165 (100 ng/mL; R&D Systems) in 1% FBS buffer with 2 μg/mL heparin at 0°C for 1 hour. Sema3A/Fc or biotinylated VEGF165 bound to cells was detected with FITC-conjugated F(ab′)2 goat anti–human IgG Fc (Jackson ImmunoResearch Laboratories, West Grove, PA) or avidin-FITC (R&D Systems). Data were collected using a FACScalibur cytofluorometer.
Endothelial cell retraction assay
HUVECs were incubated in 1% FBS buffer with or without DS500 (2 μg/mL) or fucoidan (8 μg/mL) at 37°C for 1 hour. After washing 3 times with assay medium (1% FBS buffer with 2 μg/mL heparin), HUVECs (16 000 cells/chamber) were added to the 4-chamber glass slides precoated with 5 μg/mL fibronectin. After 1 hour, cells were stimulated with Sema3A (2 μg/mL, 1 hour). After fixation with 4% wt/vol paraformaldehyde, 4 nonoverlapping fields were observed to obtain an average retraction score, as described.21
Endothelial cell proliferation assay
HUVECs (2000 cells/well) were cultured for 3 days in 96-well tissue culture plates (Corning, Corning, NY) in MEDIUM199 with 10% FBS and 25 μg/mL heparin, with or without 25 ng/mL VEGF165. Proliferation was measured by 3H thymidine uptake ([0.6 μCi/well] 0.022 MBg/well; New England Nuclear, Beverly, MA) during the last 16 hours of culture.
In vivo Matrigel and tumor angiogenesis assays
All animal experiments were approved by the NCI-Bethesda Animal Care and Use Committee. The Matrigel assay was performed essentially as described.44 Mice (female C57BL/6J and BALB/cAnNCr, 6-7 weeks old; The Jackson Laboratory, Bar Harbor, ME) were injected subcutaneously with 0.5 mL Matrigel (BD Biosciences, Bedford, MA) containing VEGF165 (150 ng/mL) plus heparin (500 ng/mL) or PBS. The tumor angiogenesis assay was carried out in female BALB/cAnNCr mice (6 weeks old). Mice were injected subcutaneously with 107 MOPC 315 mouse plasma-cell tumor line (from tumor-bearing BALB/c mice45 ). One hour after injection of Matrigel or MOPC315 tumor cells, mice were injected intraperitoneally with polysaccharides (dextran, DS500, or fucoidan 1 mg in 0.2 mL saline). Treatment was repeated daily for 6 (Matrigel assay) or 7 (tumor assay) days; on days 7 and 8, plugs and tumors were removed, respectively. Tumor size (product of maximum perpendicular caliper measurements) and weight were measured. Tissues were fixed (cold 4% paraformaldehyde in PBS), washed in 15% and 30% sucrose, embedded in OCT, and processed for histology. Sections were stained with H&E and immunostained for CD31 with purified rat anti–mouse CD31/PECAM monoclonal antibody (BD Pharmingen) followed by Alexa Fluor 647–conjugated goat anti–rat IgG (Molecular Probes) with DAPI. Sections were imaged with an Axiovert 200 fluorescence microscope (Carl Zeiss) using optimized excitation/emission filter sets (Omega Optical, Brattleboro, VT). Each labeling reaction was captured using light filtered through an appropriate filter set, and the images were digitized using the OpenLab imaging program (Improvision, Lexington, MA). An appropriate color table was applied to each image to either match its emission spectrum or to set a distinguishing color balance. The pseudocolored images were then converted into tiff files, exported to Adobe Photoshop (Adobe Systems, San Jose, CA), and overlaid as individual layers to create multicolored merged composites. Angiogenesis was evaluated by digital measurement (IPLab software; BioVision Technologies, Exton, PA) of CD31+ cells within Matrigel plugs and tumor tissues. The results are expressed as the mean surface area occupied by CD31+ cells/unit area (μm2/106 μm2).
Statistical analysis
Results are expressed as means plus or minus SD or SEM. Student t test was applied to evaluate group differences; a P value of less than .05 was considered significant.
Results
Analysis of NRP1 binding to polysaccharides
Heparin binds to the extracellular domain of NRP1 within the b1/b2 subdomain.15,27 We examined whether other polysaccharides can bind to NRP1. Using surface plasmon resonance (Biacore system), we confirmed that recombinant NRP1 extracellular domain dose dependently binds to a heparin-coated sensor chip (not shown). On the basis of the results of 3 experiments, the Kd for the NRP1-heparin interaction was calculated at 0.69 (± 0.13) nM (association rate constant [Ka]: 5.7 ± 1.1 × 106 1/Ms and dissociation rate constant [Kd]: 3.9 ± 0.19 × 10−3 1/s). Using this system, we examined the effects of various polysaccharides on the binding of NRP1 to immobilized heparin (Figure 1A). Heparin itself at 1 μg/mL and to a lesser extent at 0.1 μg/mL reduced the binding of NRP1 to heparin. Within a panel of 8 polysaccharides, dextran sulfate (DS) with Mr 500 000 Da (DS500), fucoidan (1 and 0.1 μg/mL), and DS with Mr 8000 Da (DS8) (1μg/mL) markedly reduced the binding of NRP1 to heparin. Heparan sulfate (HS), chondroitin sulfate A (ChoSul A), ChoSul B, ChoSul C, and nonsulfated dextran (all at 1 μg/mL) minimally affected the binding of NRP1 to heparin. These results demonstrate that DS500 and fucoidan inhibit NRP1 binding to heparin, raising the possibility that these polysaccharides can bind to NRP1. We looked for binding of recombinant NRP1/Fc to immobilized DS (average Mr: 5000 Da). Heparin- and maltose-gel were used as controls for DS-gel, and gp130/Fc was used as a control for NRP1/Fc. We affinity purified abundant NRP1/Fc from heparin-gel and DS-gel, but only little from control maltose-gel. We could not elute gp130/Fc from these gels (Figure 1B). These experiments demonstrate that DS directly binds to NRP1.
The sulfated polysaccharides DS500 and fucoidan interact with NRP1 and reduce cell-surface levels of NRP1 on endothelial cells. (A) Effects of polysaccharides on NRP1 binding to heparin. NRP1 (20 nM) was passed over a heparin-coated sensor chip without (black line), or with 0.1 μg/mL (blue line) or 1 μg/mL (red line) polysaccharide. (B) Binding of NRP1 to dextran sulfate. NRP1/Fc (1 μg/mL) or gp130/Fc (1 μg/mL) was incubated with heparin-gel, DS-gel, and maltose-gel and the precipitates were detected by anti-Fc antibody. NRP1/Fc or gp130/Fc (100 ng) was loaded as a control. (C) Modulation of cell-surface NRP1 by polysaccharides. HUVECs were incubated with the polysaccharides (0-64 μg/mL, 37°C, 1 hour). After cell washing (1 M NaCl), NRP1 was detected by flow cytometry. Results reflect the relative mean fluorescence intensities with and without polysaccharide. (D) Effects of DS500 on levels of cell-surface molecules NRP1, NRP2, VEGFR-1, VEGFR-2, CD31, VE-cadherin, gp130, and CXCR4. HUVECs were incubated (37°C, 1 hour) with or without DS500 (8 μg/mL). Shaded graphs reflect control staining. (E) Temperature-, concentration-, and time-dependent reduction of cell-surface NRP1 and NRP2 by DS500. HUVECs were incubated with DS500. NRP1 and NRP2 were detected by flow cytometry. (F) HUVECs were treated (37°C, 1 hour) with or without DS500 (8 μg/mL), stained for NRP1 (green) and DAPI (blue), fixed, and observed through an Olympus IX51 phase-contrast microscope equipped with a 10×/0.25 PhC objective lens and a 10× eyepiece (Olympus Optical, Melville, NY), and were photographed with a Retiga 1300 digital camera (Qimaging, Burnaby, BC). Original magnification ×100. (G) NRP1 detected on HUVECs incubated with DS500 (0-8 μg/mL, 37°C, 1 hour) in the presence of 1% or 95% human serum.
The sulfated polysaccharides DS500 and fucoidan interact with NRP1 and reduce cell-surface levels of NRP1 on endothelial cells. (A) Effects of polysaccharides on NRP1 binding to heparin. NRP1 (20 nM) was passed over a heparin-coated sensor chip without (black line), or with 0.1 μg/mL (blue line) or 1 μg/mL (red line) polysaccharide. (B) Binding of NRP1 to dextran sulfate. NRP1/Fc (1 μg/mL) or gp130/Fc (1 μg/mL) was incubated with heparin-gel, DS-gel, and maltose-gel and the precipitates were detected by anti-Fc antibody. NRP1/Fc or gp130/Fc (100 ng) was loaded as a control. (C) Modulation of cell-surface NRP1 by polysaccharides. HUVECs were incubated with the polysaccharides (0-64 μg/mL, 37°C, 1 hour). After cell washing (1 M NaCl), NRP1 was detected by flow cytometry. Results reflect the relative mean fluorescence intensities with and without polysaccharide. (D) Effects of DS500 on levels of cell-surface molecules NRP1, NRP2, VEGFR-1, VEGFR-2, CD31, VE-cadherin, gp130, and CXCR4. HUVECs were incubated (37°C, 1 hour) with or without DS500 (8 μg/mL). Shaded graphs reflect control staining. (E) Temperature-, concentration-, and time-dependent reduction of cell-surface NRP1 and NRP2 by DS500. HUVECs were incubated with DS500. NRP1 and NRP2 were detected by flow cytometry. (F) HUVECs were treated (37°C, 1 hour) with or without DS500 (8 μg/mL), stained for NRP1 (green) and DAPI (blue), fixed, and observed through an Olympus IX51 phase-contrast microscope equipped with a 10×/0.25 PhC objective lens and a 10× eyepiece (Olympus Optical, Melville, NY), and were photographed with a Retiga 1300 digital camera (Qimaging, Burnaby, BC). Original magnification ×100. (G) NRP1 detected on HUVECs incubated with DS500 (0-8 μg/mL, 37°C, 1 hour) in the presence of 1% or 95% human serum.
Effects of DS500 and fucoidan on cell-surface NRP1
Heparin binds to cell-surface NRP1 and enhances the biologic activity of VEGF165 and Sema3A.15,29 We now tested whether DS500 and fucoidan can also bind to cell-surface NRP1 and modulate its function. Primary human umbilical vein endothelial cells (HUVECs) were incubated with each of the polysaccharides tested in Figure 1A at 37°C for 1 hour, and after washing with 1 M NaCl (which effectively removes NRP1 from heparin as assessed by Biacore), we measured levels of cell-surface NRP1 by flow cytometry. As shown in Figure 1C, DS500 and to a lesser degree fucoidan reduced NRP1 levels on HUVECs, but all other polysaccharides, including heparin, did not. By the same method, DS500 reduced cell-surface NRP2 and to a lesser extent VEGFR-1 and VEGFR-2, but minimally reduced cell-surface CD31, VE-cadherin, gp130, or CXCR4 (Figure 1D), indicating that DS500 does not indiscriminately alter detection of cell-surface molecules. Similar results were derived with fucoidan (not shown). We examined the conditions for reduction of cell-surface NRP1 and NRP2 by DS500 (Figure 1E). At 37°C, but not at 0°C, DS500 dose dependently reduced cell-surface NRP1 and NRP2, with maximal inhibition at 2 μg/mL and ED50 of 1.0 (± 0.13) μg/mL (2.0 ± 0.26 nM; average of 3 experiments). This reduction occurred progressively over 90 minutes. Thus, DS500 reduces cell-surface NRP1 and NRP2 on endothelial cells in a temperature-, concentration-, and time-dependent manner. Using fluorescence microscopy, we confirmed visually that cell-surface NRP1 is reduced on HUVECs after 1-hour incubation with DS500 (8 μg/mL) at 37°C compared with control cells incubated in medium only (Figure 1F representative images). Since several serum components are known to interact with glycosaminoglycans,30 we examined the effect of human serum on the reduction of cell-surface NRP1 by DS500. As shown in Figure 1G, DS500 dose dependently reduced cell-surface NRP1 on HUVECs in the presence of 95% human serum, and the reduction was similar to that achieved in the presence of 1% human serum. Thus, DS500 reduces cell-surface NRP1 on endothelial cells even in the presence of high serum concentrations.
DS500 and fucoidan promote the internalization of cell-surface NRP1
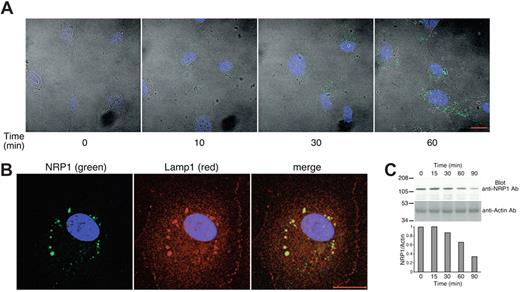
We next examined whether DS500 and fucoidan promote the internalization of NRP1; this would explain why cell-surface NRP1 is detected at lower levels after incubation with these polysaccharides. Using confocal microscopy, we traced NRP1 in endothelial cells after 10- to 60-minute incubation with DS500 (8 μg/mL) at 37°C. At time 0, NRP1 is minimally detectable in HUVECs that have been fixed and permeabilized. After 10 minutes, NRP1 staining is visible at low levels and becomes progressively more intense. After 60 minutes, NRP1 is clearly identified by a vesicular-like staining (Figure 2A), and colocalizes with Lamp1 (lysosome associated membrane protein-1; Figure 2B). Similar results were obtained with fucoidan (not shown). These observations demonstrated that DS500 promotes NRP1 internalization and trafficking to the lysosomal compartment. Western blot analysis of endothelial cell lysates showed that the intensity of the NRP1-related band decreases after incubation with DS500 longer than 30 minutes (Figure 2C), suggesting that NRP1 is degraded in the lysosomes. Together, these results demonstrate that DS500 promotes NRP1 internalization from the cell surface to the cytoplasm where it reaches the lysosome and is degraded.
DS500 promotes NRP1 internalization and colocalization with Lamp1. (A) DS500 induces NRP1 internalization. HUVECs grown on fibronectin-coated glass slides were incubated with DS500 (8 μg/mL, 37°C, 0-60 minutes). After fixation and permeabilization, cells were stained with anti-NRP1 mAb and examined by an LSM510 confocal microscope equipped with a Plan-Neofluar 40×1/1.3 objective lens (Carl Zeiss). Images reflect the merging of fluorescent slice images of NRP1 (green), DAPI (blue), and differential interference contrast image. Images were imported into Adobe Photoshop 6.0 (Adobe Systems) for processing. Scale bar represents 20 μm. (B) NRP1 colocalizes with Lamp1. HUVECs were incubated with DS500 (8 μg/mL, 37°C, 1 hour). After fixation and permeabilization, cells were stained for NRP1 (green), Lamp1 (red) and DAPI (blue), and examined by confocal microscopy. (C) DS500 reduces protein levels of NRP1. Cell lysates of HUVECs treated with DS500 (2 μg/mL, 37°C, 0-90 minutes) were blotted with anti-NRP1 Ab (top panel) and reblotted with antiactin Ab (bottom panel). Relative ratios of NRP1/actin band intensities are shown in the lower bar graph.
DS500 promotes NRP1 internalization and colocalization with Lamp1. (A) DS500 induces NRP1 internalization. HUVECs grown on fibronectin-coated glass slides were incubated with DS500 (8 μg/mL, 37°C, 0-60 minutes). After fixation and permeabilization, cells were stained with anti-NRP1 mAb and examined by an LSM510 confocal microscope equipped with a Plan-Neofluar 40×1/1.3 objective lens (Carl Zeiss). Images reflect the merging of fluorescent slice images of NRP1 (green), DAPI (blue), and differential interference contrast image. Images were imported into Adobe Photoshop 6.0 (Adobe Systems) for processing. Scale bar represents 20 μm. (B) NRP1 colocalizes with Lamp1. HUVECs were incubated with DS500 (8 μg/mL, 37°C, 1 hour). After fixation and permeabilization, cells were stained for NRP1 (green), Lamp1 (red) and DAPI (blue), and examined by confocal microscopy. (C) DS500 reduces protein levels of NRP1. Cell lysates of HUVECs treated with DS500 (2 μg/mL, 37°C, 0-90 minutes) were blotted with anti-NRP1 Ab (top panel) and reblotted with antiactin Ab (bottom panel). Relative ratios of NRP1/actin band intensities are shown in the lower bar graph.
DS500 and fucoidan promote the internalization of cell-surface SREC-I
We investigated the mechanisms underlying NRP1 internalization induced by DS500. First, we tested whether binding of DS500 to cell-surface NRP1 is sufficient for internalization. Besides HUVECs, NRP1 is detected on the human leukemia RS4 and the human stromal HS-5 cell lines, and on COS7 cells transduced with human NRP1 (COS7-NRP1) (Figure 3A). DS500 dose-dependently reduced cell-surface NRP1 on HUVECs, but failed to do so on RS4, HS-5, or COS7-NRP1 cells (Figure 3B), indicating that NRP1 internalization by DS500 requires additional critical components, which are present in HUVECs but not in the other cell types tested. One of the parameters that we found distinguishes HUVECs from RS4, HS-5, and COS7-NRP1 is expression of scavenger receptors, which mediate the uptake of LDL modified by acetylation or oxidation. Some of the scavenger receptors are known to interact with sulfated polysaccharides.32-34 As shown in Figure 3C, HUVECs displayed a dose-dependent uptake of DiO-Ac-LDL, whereas RS4, HS-5, and COS7-NRP1 displayed only minimal uptake. Unlabeled Ac-LDL blocked uptake of DiO-Ac-LDL in HUVECs, indicating the Ac-LDL specificity of DiO-Ac-LDL uptake in these cells.
Cell specificity of NRP1 reduction by DS500. (A) Cell-surface NRP1 in HUVECs, RS4, HS-5, and COS7-NRP1 cells analyzed by flow cytometry. (B) Reduction of cell-surface NRP1 is detected on HUVECs, but not RS4, HS-5, and COS7-NRP1 cells after stimulation with DS500 (0, 0.5, 2, 8 μg/mL, 37°C, 1 hour). Results reflect the relative mean fluorescence intensities with and without stimulation. (C) Uptake of DiO-Ac-LDL (0, 0.25, 1, 4 μg/mL, 37°C, 1 hour) by HUVECs, but not RS4, HS-5, and COS7-NRP1 cells detected by flow cytometry. Open circles indicate cell uptake of DiO-Ac-LDL (4 μg/mL) in the presence of competitor Ac-LDL (100 μg/mL). Results reflect mean fluorescence intensities after background subtraction.
Cell specificity of NRP1 reduction by DS500. (A) Cell-surface NRP1 in HUVECs, RS4, HS-5, and COS7-NRP1 cells analyzed by flow cytometry. (B) Reduction of cell-surface NRP1 is detected on HUVECs, but not RS4, HS-5, and COS7-NRP1 cells after stimulation with DS500 (0, 0.5, 2, 8 μg/mL, 37°C, 1 hour). Results reflect the relative mean fluorescence intensities with and without stimulation. (C) Uptake of DiO-Ac-LDL (0, 0.25, 1, 4 μg/mL, 37°C, 1 hour) by HUVECs, but not RS4, HS-5, and COS7-NRP1 cells detected by flow cytometry. Open circles indicate cell uptake of DiO-Ac-LDL (4 μg/mL) in the presence of competitor Ac-LDL (100 μg/mL). Results reflect mean fluorescence intensities after background subtraction.
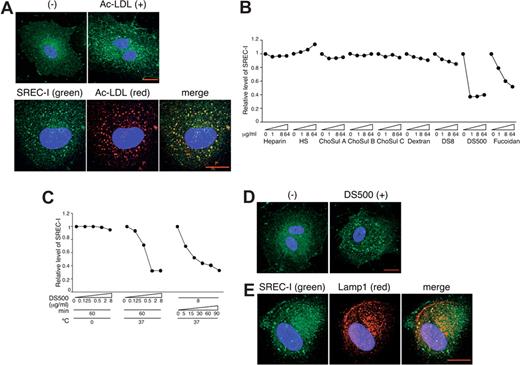
Several scavenger receptors have been identified in endothelial cells.32 We focused on SREC-I (scavenger receptor expressed by endothelial cells-I), as it is highly expressed on HUVECs, and examined whether SREC-I can mediate the uptake of Ac-LDL in HUVECs. By confocal microscopy, we found that Ac-LDL promotes the intracellular accumulation of SREC-I (green) (Figure 4A top panels), and that internalized SREC-I (green) and Ac-LDL (red) colocalize (yellow) at least in part within HUVECs (Figure 4A bottom panels). This provides evidence that SREC-I can mediate the uptake of Ac-LDL in HUVECs, and that SREC-I itself is internalized after binding to its ligand.
Sulfated polysaccharides reduce cell-surface levels of SREC-I, and promote SREC-I internalization and colocalization with Lamp1. (A) SREC-I internalized by Ac-LDL localizes with Ac-LDL. SREC-I (green) and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with Alexa 594–conjugated Ac-LDL (red) (4 μg/mL, 37°C, 1 hour), fixed, and permeabilized. Scale bar represents 20 μm. Images were acquired and processed as described for Figure 3A. (B) DS500 and fucoidan reduce cell-surface levels of SREC-I. HUVECs were incubated with polysaccharides (0-64 μg/mL, 37°C, 1 hour) and washed (1 M NaCl). SREC-I was detected by flow cytometry. Results reflect relative mean fluorescence intensities with or without stimulation. (C) Temperature-, concentration-, and time-dependent reduction of cell-surface SREC-I by DS500. HUVECs were incubated with DS500. SREC-I was detected by flow cytometry. (D) DS500 induces SREC-I internalization. SREC-I (green) and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with DS500 (8 μg/mL, 37°C, 1 hour), fixed, and permeabilized. Scale bar represents 20 μm. (E) SREC-I colocalizes with Lamp1. SREC-I (green), Lamp1 (red), and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with DS500 (8 μg/mL, 37°C, 1 hour), fixed, and permeabilized.
Sulfated polysaccharides reduce cell-surface levels of SREC-I, and promote SREC-I internalization and colocalization with Lamp1. (A) SREC-I internalized by Ac-LDL localizes with Ac-LDL. SREC-I (green) and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with Alexa 594–conjugated Ac-LDL (red) (4 μg/mL, 37°C, 1 hour), fixed, and permeabilized. Scale bar represents 20 μm. Images were acquired and processed as described for Figure 3A. (B) DS500 and fucoidan reduce cell-surface levels of SREC-I. HUVECs were incubated with polysaccharides (0-64 μg/mL, 37°C, 1 hour) and washed (1 M NaCl). SREC-I was detected by flow cytometry. Results reflect relative mean fluorescence intensities with or without stimulation. (C) Temperature-, concentration-, and time-dependent reduction of cell-surface SREC-I by DS500. HUVECs were incubated with DS500. SREC-I was detected by flow cytometry. (D) DS500 induces SREC-I internalization. SREC-I (green) and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with DS500 (8 μg/mL, 37°C, 1 hour), fixed, and permeabilized. Scale bar represents 20 μm. (E) SREC-I colocalizes with Lamp1. SREC-I (green), Lamp1 (red), and DAPI (blue) were examined by confocal microscopy in HUVECs incubated with DS500 (8 μg/mL, 37°C, 1 hour), fixed, and permeabilized.
Certain sulfated polysaccharides can bind to selected scavenger receptors and block Ac-LDL uptake.32-34 We therefore examined whether DS500, fucoidan, and other sulfated polysaccharides could serve as ligands for SREC-I. Using fluorescence-activated cell sorting (FACS) analysis for detection of cell-surface SREC-I, we found that DS500 and to a lesser degree fucoidan reduced cell-surface SREC-I in HUVECs when incubated for 1 hour at 37°C, whereas heparin, HS, ChoSul A, ChoSul B, ChoSul C, and nonsulfated dextran did not (Figure 4B). VEGF165 reduced cell-surface NRP1 but not SREC-I in HUVECs (data not shown). Reduction of cell-surface SREC-I induced by DS500 in HUVECs was temperature, dose, and time dependent (Figure 4C). We next examined whether DS500, like Ac-LDL, can promote the internalization of SREC-I in endothelial cells. After HUVECs were incubated with DS500 for 1 hour at 37°C, SREC-I displayed a vesicular-like cytoplasmic staining pattern, indicative of SREC-I internalization (Figure 4D). Similar results were derived by incubation with fucoidan (data not shown). The internalized SREC-I colocalized in part with Lamp1, indicative of lysosomal localization (Figure 4E).
Sulfated polysaccharides bridge the extracellular domain of NRP1 with that of SREC-I and induce the coordinate internalization of NRP1 and SREC-I
Since DS500 and fucoidan selectively promote NRP1 and SREC-I internalization in HUVECs (Figures 1,4) under remarkably similar conditions (concentration, temperature, and length of incubation), we tested whether the 2 polysaccharide-induced effects might be linked. After incubation with polysaccharides (37°C, 1 hour) and permeabilization, we found that DS500 and fucoidan, but not heparin or ChoSul A, promoted the internalization of SREC-I and NRP1, and importantly, we found that internalized NRP1 and SREC-I colocalize in structures (Figure 5A right panels, yellow), which we have identified as lysosomes (Figures 2B and 4E). We tested directly whether DS500 can bridge SREC-I and NRP1 using an enzyme-linked immunosorbent assay (ELISA)-based assay in which SREC-I/Fc or control IgG1 is immobilized onto the well and NRP1/Fc is then added with DS500 at various concentrations. As shown in Figure 5B, we found that DS500 dose-dependently promotes the binding of NRP1 to SREC-I (open circles), but not to IgG1 (closed circles); maximal NRP1 binding to SREC-I occurred at the DS500 concentration of 500 ng/mL (Figure 6B top panel). Using DS500 at 500 ng/mL, the binding of NRP1 to immobilized SREC-I was dependent on NRP-1 concentration (Figure 5B bottom left panel); control B7-1/Fc minimally bound to SREC-I or IgG1 (Figure 5B bottom right panel). Among the polysaccharides tested, DS500 and to a lesser degree fucoidan promoted NRP1 binding to SREC-I, whereas the other polysaccharides were minimally effective (Figure 5C). Together, these results indicate that DS500 and fucoidan can bridge NRP1 to SREC-I, and induce their coordinate internalization from the endothelial cell surface to the cytoplasm where NRP1 and SREC-I colocalize.
Analysis of NRP1 and SREC-I colocalization and association in the presence of sulfated polysaccharides. (A) Colocalization of SREC-I and NRP1 in the cytoplasm after fucoidan or DS500 stimulation. HUVECs were incubated (37°C, 1 hour) with medium alone, heparin, ChoSul A, fucoidan, or DS500 (8 μg/mL). After fixation and permeabilization, cells were stained for SREC-I (red), NRP1 (green), and DAPI (blue), and examined by confocal microscopy. Scale bar represents 20 μm. Images were acquired and processed as described for Figure 2A. (B) DS500 specifically and dose-dependently promotes binding of NRP1 to SREC-I. NRP1/Fc or control Fc protein (B7-1/Fc) was added to control IgG1-coated wells (●) or SREC-I/Fc–coated wells (○) with or without DS500. Bound NRP1 or control/Fc was measured by ELISA. The results reflect the means (± SD) of 3 experiments. (C) Effect of polysaccharides on the binding of NRP1 to SREC-I. NRP1/Fc (2μg/mL) was added to SREC-I/Fc–coated wells in the presence of the indicated polysaccharide (500 ng/mL). Bound NRP1/Fc was measured by ELISA. The results reflect the means (± SD) of 3 experiments. (D) Transduction of 293 cells with SREC-I confers DiO-Ac-LDL uptake capability and reduces cell-surface levels of NRP1, but not levels of gp130 or CXCR4. 293 cells were transfected with cDNA for SREC-I (red line) or control (blue line). Uptake of DiO-Ac-LDL and cell-surface levels of endogenous gp130, CXCR4, or NRP1 were detected by flow cytometry. Shaded graphs reflect control staining.
Analysis of NRP1 and SREC-I colocalization and association in the presence of sulfated polysaccharides. (A) Colocalization of SREC-I and NRP1 in the cytoplasm after fucoidan or DS500 stimulation. HUVECs were incubated (37°C, 1 hour) with medium alone, heparin, ChoSul A, fucoidan, or DS500 (8 μg/mL). After fixation and permeabilization, cells were stained for SREC-I (red), NRP1 (green), and DAPI (blue), and examined by confocal microscopy. Scale bar represents 20 μm. Images were acquired and processed as described for Figure 2A. (B) DS500 specifically and dose-dependently promotes binding of NRP1 to SREC-I. NRP1/Fc or control Fc protein (B7-1/Fc) was added to control IgG1-coated wells (●) or SREC-I/Fc–coated wells (○) with or without DS500. Bound NRP1 or control/Fc was measured by ELISA. The results reflect the means (± SD) of 3 experiments. (C) Effect of polysaccharides on the binding of NRP1 to SREC-I. NRP1/Fc (2μg/mL) was added to SREC-I/Fc–coated wells in the presence of the indicated polysaccharide (500 ng/mL). Bound NRP1/Fc was measured by ELISA. The results reflect the means (± SD) of 3 experiments. (D) Transduction of 293 cells with SREC-I confers DiO-Ac-LDL uptake capability and reduces cell-surface levels of NRP1, but not levels of gp130 or CXCR4. 293 cells were transfected with cDNA for SREC-I (red line) or control (blue line). Uptake of DiO-Ac-LDL and cell-surface levels of endogenous gp130, CXCR4, or NRP1 were detected by flow cytometry. Shaded graphs reflect control staining.
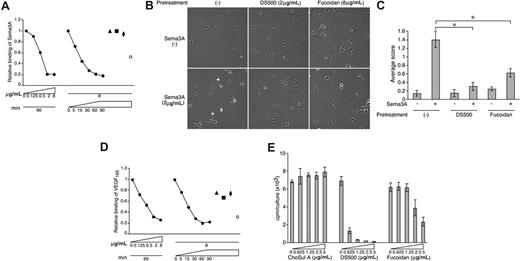
DS500 and fucoidan inhibit Sema3A and VEGF165 cell binding and function. (A) DS500 and fucoidan block Sema3A binding to HUVECs. Cells were incubated with DS500 (●; 0-8 μg/mL), heparin (▲), ChoSul A (■), dextran (♦), or fucoidan (○; 8 μg/mL), washed (1 M NaCl), and incubated with Sema3A/Fc. Bound Sema3A/Fc was detected by flow cytometry. (B,C) DS500 and fucoidan inhibit Sema3A-induced lamellipodia retraction in HUVECs. After preincubation with or without DS500 or fucoidan, HUVECs were allowed to attach onto fibronectin-coated slides, and then incubated with or without Sema3A/Fc. (B) Representative images. Magnification 100×. (C) Average retraction scores (± SD of 4 fields). *P < .01. (D) DS500 and fucoidan block VEGF165 binding to HUVECs. Bound VEGF165was detected by flow cytometry. Experimental conditions as described in panel A. (E) DS500 and fucoidan inhibit VEGF165-induced proliferation of HUVECs. Cells were cultured (3 days) with ChoSul A, DS500, or fucoidan in the presence of VEGF165 (25 ng/mL); proliferation was measured by 3H-thymidine uptake. Results are expressed as mean cpm/culture (± SD of triplicate cultures).
DS500 and fucoidan inhibit Sema3A and VEGF165 cell binding and function. (A) DS500 and fucoidan block Sema3A binding to HUVECs. Cells were incubated with DS500 (●; 0-8 μg/mL), heparin (▲), ChoSul A (■), dextran (♦), or fucoidan (○; 8 μg/mL), washed (1 M NaCl), and incubated with Sema3A/Fc. Bound Sema3A/Fc was detected by flow cytometry. (B,C) DS500 and fucoidan inhibit Sema3A-induced lamellipodia retraction in HUVECs. After preincubation with or without DS500 or fucoidan, HUVECs were allowed to attach onto fibronectin-coated slides, and then incubated with or without Sema3A/Fc. (B) Representative images. Magnification 100×. (C) Average retraction scores (± SD of 4 fields). *P < .01. (D) DS500 and fucoidan block VEGF165 binding to HUVECs. Bound VEGF165was detected by flow cytometry. Experimental conditions as described in panel A. (E) DS500 and fucoidan inhibit VEGF165-induced proliferation of HUVECs. Cells were cultured (3 days) with ChoSul A, DS500, or fucoidan in the presence of VEGF165 (25 ng/mL); proliferation was measured by 3H-thymidine uptake. Results are expressed as mean cpm/culture (± SD of triplicate cultures).
To further define the role of SREC-I in NRP1 internalization, we expressed SREC-I in human 293 cells, which do not express endogenous scavenger receptors, but express gp130, CXCR4, and NRP1. As shown in Figure 5D, control 293 cells did not uptake DiO-Ac-LDL, but 293-SREC-I cells could uptake DiO-Ac-LDL, indicative of SREC-I function (top left). An analysis of cell-surface gp130, CXCR4, and NRP1 showed that levels of gp130 (Figure 5D top right) and CXCR4 (bottom left) were similar in control and SREC-I–transfected 293 cells. By contrast, cell-surface NRP1 was significantly reduced in 293-SREC-I cells compared with control 293 cells (Figure 5D bottom right). DS500 induced only minimal further reduction of cell-surface NRP1 levels in 293-SREC-I cells (data not shown), suggesting that forced expression of SREC-I alone reduces cell-surface NRP1 in this experimental system.
Treatment of endothelial cells with DS500 or fucoidan blocks Sema3A and VEGF165 function
We examined the potential functional significance of the reduction of NRP1, NRP2, VEGFR-1, and VEGFR-2 induced by DS500 and fucoidan (Figure 1C-E). As shown in Figure 6A, pretreatment with DS500 followed by cell washing dose- and time-dependently inhibited the binding of Sema3A to HUVECs. Instead, pretreatment with heparin, ChoSul A, and dextran (all at 8 μg/mL for 90 minutes) did not affect binding of Sema3A to HUVECs (Figure 6A filled triangle, square, and diamond). Fucoidan also inhibited the binding of Sema3A to HUVECs (Figure 6A open circle). Endothelial cells spread lamellipodia when placed onto a fibronectin-coated glass surface, and Sema3A is known to induce retraction of these lamellipodia.21 We found that Sema3A induces minimal retraction of lamellipodia in HUVECs that were pretreated with DS500 or fucoidan, indicative that these polysaccharides can block this function of Sema3A (Figure 6B). We scored the degree of retraction in 3 independent experiments, as described,21 and found that DS500 and fucoidan reduce significantly Sema3A-induced lamellipodia retraction in HUVECs (Figure 6C). We also found that pretreatment with DS500 dose- and time-dependently blocked the binding of VEGF165 to HUVECs, whereas pretreatment with heparin, ChoSul A, and dextran did not affect VEGF165 binding to these cells (Figure 6D). Fucoidan also inhibited VEGF165 binding to HUVECs (Figure 6D). Thus, DS500, fucoidan, heparin, ChoSul A, and dextran have parallel effects on the binding of Sema3A and VEGF165 to HUVECs (Figure 6A,D). We examined the effects of polysaccharides on VEGF165-induced proliferation of endothelial cells. As shown in Figure 6E, DS500 and to a lesser degree fucoidan inhibited HUVEC proliferation in response to VEGF165, whereas ChoSul A was minimally effective.
Fucoidan inhibits angiogenesis in vivo
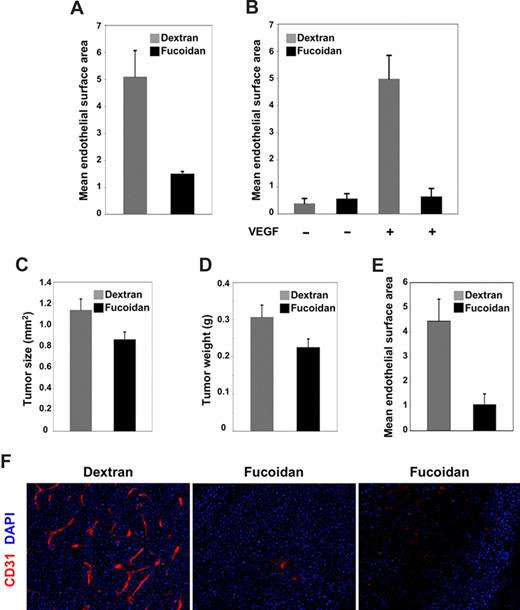
We tested the effects of fucoidan and DS500 on VEGF-induced angiogenesis in vivo. To this end, we established subcutaneous Matrigel plugs containing VEGF (150 ng/mL) plus heparin (0.5 μg/mL) or PBS alone in mice (C57BL/6J, 7 weeks old), and then treated groups of these mice (5 mice/group) intraperitoneally with fucoidan, DS500, or dextran (all at 1 mg/mouse) daily for 6 days beginning on the day of Matrigel injection. DS500 treatment induced death in 8 of 10 animals; fucoidan and dextran were well tolerated. All plugs were removed from the mice on day 7 and processed for histology and immunohistochemistry (CD31/PCAM staining). The endothelial cell density (measured digitally with IPLab software) was significantly (P = .003) reduced in VEGF-supplemented plugs from mice treated with fucoidan compared with dextran (Figure 7A). Plugs without VEGF supplementation displayed minimal cell infiltration with or without systemic treatment (not shown). In a repeat experiment using BALB/cAnNCr mice (6 weeks old; 6 mice/group), we confirmed that Matrigel plugs from the fucoidan-treated group contained a significantly (P < .001) reduced endothelial cell infiltration compared with the dextran-treated group (Figure 7B), providing evidence that fucoidan inhibits VEGF-induced neovascularization.
Fucoidan inhibits angiogenesis in vivo. (A,B) Effects of dextran and fucoidan on VEGF-induced angiogenesis in Matrigel plugs. Mice bearing Matrigel plugs containing VEGF plus heparin or PBS alone were injected daily intraperitoneally for 6 days with dextran or fucoidan (1 mg/mouse per day). After the plugs were fixed and immunostained for CD31/PECAM, endothelial cell density was evaluated microscopically (Nikon Eclipse E600) equipped with a DIC M 20×/0.75 Nikon lens (Nikon, Tokyo, Japan). Images were imported into IPLab software, and the area occupied by CD31+ cells was quantified. The results are expressed as mean (± SD) surface areas (μm2) occupied by CD31+ cells within a unit area (106 μm2). In panel A, the 2 groups consisted of 5 C57BL/6J 7-week-old female mice; in panel B, the 4 groups consisted 6 BALB/cAnNCr 6-week-old female mice. (C,D) Effects of dextran and fucoidan on tumor growth in mice. Mean (± SEM) (C) tumor size (expressed in square millimeters) and (D) tumor weight (expressed in grams) in the 2 groups of 15 female BALB/cAnNCr 6-week-old mice inoculated subcutaneously with 107 MOPC315 tumor cells and subsequently treated daily for 7 days with dextran or fucoidan. (E) Vascular infiltration in tumor tissues from mice treated with dextran or fucoidan was quantified using IPLab software after immunohistochemical staining for CD31. The results are expressed as the mean (± SD) surface areas occupied by CD31+ cells within a unit area (μm2/106 μm2). (F) Representative images reflecting CD31/PECAM immunostaining of tumor tissues from mice treated with dextran (left panel) or fucoidan (middle and right panel). CD31 is displayed in red; DAPI nuclear staining in blue. Original magnification 20×.
Fucoidan inhibits angiogenesis in vivo. (A,B) Effects of dextran and fucoidan on VEGF-induced angiogenesis in Matrigel plugs. Mice bearing Matrigel plugs containing VEGF plus heparin or PBS alone were injected daily intraperitoneally for 6 days with dextran or fucoidan (1 mg/mouse per day). After the plugs were fixed and immunostained for CD31/PECAM, endothelial cell density was evaluated microscopically (Nikon Eclipse E600) equipped with a DIC M 20×/0.75 Nikon lens (Nikon, Tokyo, Japan). Images were imported into IPLab software, and the area occupied by CD31+ cells was quantified. The results are expressed as mean (± SD) surface areas (μm2) occupied by CD31+ cells within a unit area (106 μm2). In panel A, the 2 groups consisted of 5 C57BL/6J 7-week-old female mice; in panel B, the 4 groups consisted 6 BALB/cAnNCr 6-week-old female mice. (C,D) Effects of dextran and fucoidan on tumor growth in mice. Mean (± SEM) (C) tumor size (expressed in square millimeters) and (D) tumor weight (expressed in grams) in the 2 groups of 15 female BALB/cAnNCr 6-week-old mice inoculated subcutaneously with 107 MOPC315 tumor cells and subsequently treated daily for 7 days with dextran or fucoidan. (E) Vascular infiltration in tumor tissues from mice treated with dextran or fucoidan was quantified using IPLab software after immunohistochemical staining for CD31. The results are expressed as the mean (± SD) surface areas occupied by CD31+ cells within a unit area (μm2/106 μm2). (F) Representative images reflecting CD31/PECAM immunostaining of tumor tissues from mice treated with dextran (left panel) or fucoidan (middle and right panel). CD31 is displayed in red; DAPI nuclear staining in blue. Original magnification 20×.
We also examined the effects of fucoidan and dextran on tumor angiogenesis, and selected an experimental tumor model in which the murine plasma-cell tumor line MOPC 315 (BALB/c-derived; 107 cells/mouse), which expresses VEGF,46 is inoculated subcutaneously into BALB/cAnNCr mice (6 weeks old). We established that under these experimental conditions, MOPC315 rapidly give rise to progressively growing and highly vascularized tumors at the injection site. Using this model, we treated groups of mice (15 mice/group) with either fucoidan or dextran (1 mg/mouse intraperitoneally per day beginning on the day of tumor cell inoculation), and evaluated tumor size and vascularization on day 8. The mean (± SEM) size (1.139 ± 0.1 mm2) and weight (0.31 ± 0.03 g) of tumors removed from the 15 mice treated with dextran was significantly (P = .032 and P = .047, respectively) greater than the mean size (0.86 ± 0.07 mm2) and weight (0.23 ± 0.02 g) of tumors from the 15 fucoidan-treated mice (Figure 7C,D). Tumor tissues from dextran-treated mice were significantly (P = .007) more vascular than those from fucoidan-treated mice (Figure 7E), as revealed by quantitative analysis of CD31+ areas. Representative images reflecting immunohistochemical staining for CD31 (red) and nuclei (blue) of tumor sections from dextran- or fucoidan-treated mice are shown in Figure 7F. Thus, these results indicate that fucoidan reduces angiogenesis in distinct in vivo model systems.
Discussion
We show that the sulfated polysaccharides dextran sulfate and fucoidan reduce endothelial cell-surface levels of NRP1, NRP2, and to a lesser extent VEGFR-1 and VEGFR-2, and block the binding and function of semaphorin3A and VEGF165. We find that dextran sulfate and fucoidan can bridge the extracellular domain of NRP1 to that of the scavenger receptor SREC-I, and promote their coordinate internalization in endothelial cells. These results provide evidence that certain polysaccharides can induce internalization of selected cell-surface receptors, and delineate a strategy for dampening cell responses to undesired specific ligands.
Internalization is one of the mechanisms by which cell-surface receptors attenuate ligand-induced signaling. After a ligand binds to its receptor on the cell membrane, a signal is generated and the receptor is internalized. This sequestration of the receptor away from the cell surface often results in ligand desensitization and protection from prolonged or excessive signaling.47-49 In the case of shared-type receptors (ie, when the same cell-surface molecule is a receptor for distinct ligands) internalization of the receptor induced by one ligand can serve to block receptor binding by the second ligand. CXCR4 and CCR5 are chemokine receptors, which also serve as coreceptors for HIV-1 facilitating viral entry into target cells. SDF-1, RANTES, and some variants of these chemokines, which bind to their respective cognate CXCR4 and CCR5 receptors and induce their internalization, can inhibit HIV infection.50-52 NRP1 and NRP2 are receptors shared by VEGF members and class 3 semaphorins. VEGF-induced internalization of NRP1 or NRP2 prevents the binding of Sema3A or Sema3F.21,22 These examples are analogous to polysaccharide-induced NRP1 internalization described here, which can block the binding of NRP1 ligands Sema3A and VEGF165. Internalization of NRP1 by the sulfated polysaccharides required the presence of a scavenger receptor, which can be viewed as a necessary driver for NRP1 internalization. This is analogous to VEGF-induced internalization of NRPs, which requires VEGFRs.21,22
SREC-I binds Ac-LDL, fucoidan, and dextran sulfate.39 We now found that DS500 and fucoidan promote internalization and trafficking of SREC-I to the lysosome. We also found that SREC-I internalization, induced by these polysaccharides, is accompanied by internalization of NRP1, and that these 2 receptors are subsequently detected in the lysosome, where we suspect they are degraded. Since HUVECs express a number of scavenger receptors, it is possible that scavenger receptors other than SREC-I can mediate NRP1 internalization by sulfated polysaccharides. Nonsulfated dextran, dextran sulfate with Mr 8000 Da (DS8), and low-molecular-weight (5000 Da) fucoidan53 (not shown) did not reduce cell-surface NRP1 levels in endothelial cells, indicative that both sulfation and size are important for NRP1 internalization. Concordantly, we found that neither dextran nor DS8 could induce internalization of SREC-I or bridge NRP1 to SREC-I. Heparin binds to NRP1, but is not a ligand for SREC-I or other scavenger receptors.31 Concordantly, heparin did not reduce cell-surface NRP1. When overexpressed in 293 cells, SREC-I induced a selective reduction of NRP1 but not other surface receptors, without addition of sulfated polysaccharides. We suspect that the experimental conditions for transfection may have induced scavenger receptors bridging to NRP1 substituting for the polysaccharides, or that overexpressed SREC-I may directly interact with NRP1.
NRP1 neutralization by antibodies or by a soluble form of NRP1 was previously shown to inhibit tumor angiogenesis and tumor growth in vivo.26,54 In one study, fucoidan displayed antiangiogenic and antitumor activity in vivo, but the underlying mechanisms were not elucidated.55 We have confirmed here that fucoidan inhibits VEGF-induced and tumor angiogenesis in vivo, and provide evidence that reduction of cell-surface NRP1, NRP2, and other VEGF receptors contributes to these effects. Reduction of surface molecules not tested here and other factors may contribute to the antiangiogenic effects of polysaccharides. Fucoidan was reported to induces mobilization of stem/progenitor cells, which was attributed to competitive displacement of SDF-1 from heparan sulfate proteoglycans,56 and the sulfated polysaccharides heparin and heparan sulfate were reported to have anti-inflammatory activity, which was attributed to blockade of P-selectin– and L-selectin–mediated cell adhesion.57,58 Competitive displacement of molecules from cell-surface proteoglycans or extracellular matrix, and inhibition of cell adhesion may have contributed to the antiangiogenic effects of fucoidan.
Induction of selective internalization of surface molecules illustrates a strategy for dampening biologic effects that are dependent upon the cell-surface residence of that molecule. More classical inhibitors, such as antibodies to ligands or receptors, derive their activity from greater affinity for either ligand or receptor than the respective receptors or ligands. “Internalization inducers” derive their activity from their ability to promote receptor sequestration away from the cell surface. They need not necessarily display higher affinity than the natural ligand for effectiveness, and their cell targeting specificity is enhanced by a requirement for dual receptor recognition. In the case described here, the desired target was endothelial cell–associated NRP1. By constraining the target NRP1 with the endothelial-specific scavenger receptor, SREC-I, we selectively targeted NRP1 on endothelial cells, at the exclusion of other NRP1-expressing cell types.
By modification of natural ligands or engineering heterotypic antibodies that recognize both a target receptor and a scavenger receptor (or other receptors with similar internalization capability), various types of “internalization inducers” could be designed to sequester receptor molecules away from their natural ligands, thereby reducing ligand-induced receptor signaling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Peter J. McCormick and Ombretta Salvucci for helpful suggestions; Ms Susan Garfield for technical assistance with confocal microscopy; and Dr D. Letourneur for sharing low-molecular-weight fucoidan.
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
National Institutes of Health
Authorship
Contribution: M.N. and G.T. designed research, analyzed data, and wrote the paper; M.N. and M.S. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanna Tosato, Laboratory of Cellular Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health; Bldg 37, Rm 4124, Bethesda, MD 20892-1907; e-mail: tosatog@mail.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal