Abstract
Previous studies revealed that mAb BB9 reacts with a subset of CD34+ human BM cells with hematopoietic stem cell (HSC) characteristics. Here we map BB9 expression throughout hematopoietic development and show that the earliest definitive HSCs that arise at the ventral wall of the aorta and surrounding endothelial cells are BB9+. Thereafter, BB9 is expressed by primitive hematopoietic cells in fetal liver and in umbilical cord blood (UCB). BB9+CD34+ UCB cells transplanted into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice contribute 10-fold higher numbers of multilineage blood cells than their CD34+BB9− counterparts and contain a significantly higher incidence of SCID-repopulating cells than the unfractionated CD34+ population. Protein microsequencing of the 160-kDa band corresponding to the BB9 protein established its identity as that of somatic angiotensin-converting enzyme (ACE). Although the role of ACE on human HSCs remains to be determined, these studies designate ACE as a hitherto unrecognized marker of human HSCs throughout hematopoietic ontogeny and adulthood.
Introduction
Blood cell formation is a tightly regulated process supported by an ordered hierarchy of hematopoietic progenitor cells with a small cohort of multipotent hematopoietic stem cells (HSCs) at its apex.1 The biologic properties of HSCs are of considerable interest since these cells form the basis of one the most widely practiced forms of stem-cell therapy, bone marrow transplantation. Underpinning the clinical applications of HSCs in a wide range of cellular therapies is the ability to identify and prospectively isolate HSCs from human hematopoietic tissues. In mice, these rare cells are readily identified by the absence of mature leukocyte differentiation markers (Lin−) but express cell-surface antigens Sca-1 and c-Kit and low levels of Thy-1 (CD90).2 More recent reports have demonstrated near-homogeneous murine HSC isolation using antibodies to combinations of SLAM receptor family antigens.3 A considerable body of evidence demonstrates that HSCs in the adult human are largely restricted to a subpopulation of Lin− mononuclear characterized by coexpression of CD133, the sialomucin CD34, and Thy-1 (CD90), and corresponding low to undetectable levels of CD38.4 Another study, however, has provided evidence for the existence of putatively more primitive classes of HSCs in adulthood and during hematopoietic development that lack expression of CD34.5 The lack of a positive marker for human HSCs has thus hindered the study of all classes of HSCs and illustrates the need to identify additional cellsurface molecules on primitive hematopoietic cells to allow further characterization of candidate HSCs in human tissues.
We previously described a monoclonal antibody (mAb) BB9, that reacts with a subpopulation of CD34+ cells in human adult BM and mobilized peripheral blood.6 We now show that BB9 reacts with primitive hematopoietic cells during all stages of hematopoietic ontogeny including umbilical cord blood (UCB) and the fetal liver, and also identifies cells in the aorta-gonad mesonephros (AGM) region of the 4- to 5-week human embryo, the window in gestation that encompasses the production of the first multipotent, hematopoietic cells that constitute the stem cells of the fetal and postnatal blood systems.7-10
Finally, we demonstrate that the BB9 monoclonal antibody identifies the somatic form of angiotensin-converting enzyme (ACE/CD143), a key regulator of the renin-angiotensin system (RAS) best known for its role in the production of the vasoconstrictor and mitogen angiotensin II.11,12 Many decades of research into the RAS have elucidated new physiologic and pathophysiologic actions of this endocrine system, expanding its role not only in hypertension and atherosclerosis but also in diabetes, cardiology, oncology, and the fields of renal and reproductive physiology.13,14 Our data not only demonstrate ACE/CD143 as a newly identified cell-surface marker of human HSCs but also suggest that this membrane-bound peptidyl dipeptidase plays a previously unrecognized role in regulation of primitive hematopoietic cells throughout the ontogeny of the hematopoietic system.
Methods
Cells and tissues
Umbilical cord blood (UCB) was obtained from the Mercy Hospital for Women and Children (East Melbourne, Australia) in accordance with procedures approved by the Mercy Hospital human ethics committee. Light density UCB mononuclear cells (UCBMNCs) were isolated by separation on a Ficoll-Hypaque density gradient (Pharmacia Biotech, Uppsala, Sweden). Human fetal liver (FL) cells were purchased from Poietic Technologies (Gaithersburg, MD). Human embryonic tissues were obtained from voluntary or therapeutic abortions performed according to the guidelines and with the approval of the French National Ethic Committee. In all cases, pregnant women gave written consent for clinical procedure and research use of the embryonic tissues. Embryonic developmental age was estimated using previously described anatomic criteria.7,15
Immunolabeling for flow cytometric analysis
Flow cytometric analysis of UCBMNCs and FL cells was performed on a LSRII analyzer (Becton Dickinson, Franklin Lakes, NJ) following cell incubation with antibodies as described previously.6 Unlabeled antibodies BB9 and isotype 1B5 were used at 10 μg/mL. Biotinylated goat anti–mouse IgG1 (Southern Biotechnology, Birmingham, AL), streptavidin-APC (Becton Dickinson), CD34 (HPCA-2-FITC), CD38 (Leu17-PE), CD90 (5E10-PE), mouse IgG1-PE control (Becton Dickinson), and CD133-PE (Miltenyi Biotech, Bergisch Gladbach, Germany) antibodies were used at optimal concentrations. Addition of Fluoro Gold (Molecular Probes, Eugene, OR) excluded nonviable cells.
CD34+ cell purification and sorting
UCBMNCs were enriched for CD34+ cells using immunomagnetic beads and CD34-detach-a-bead reagent as recommended (Dynal, Oslo, Norway). CD34+ cell–enriched UCBMNCs were labeled with anti-CD34 Ab 43A116 (mouse IgG3; provided by Dr H.-J. Buhring [University of Tubingen]) and with BB9 or 1B5 isotype control Ab. BB9 and 43A1 labeling were revealed with anti–mouse IgG1-PE and anti–mouse-IgG3-FITC (Caltag, San Francisco, CA), respectively. Fluorescence-activated cell sorting (FACS) was performed on a FACS Vantage flow cytometer (Becton Dickinson) equipped with a 5-W argon ion laser emitting 488 nm light at 200 mW. Each cell fraction was reanalyzed to establish purity prior to transplantation.
NOD/SCID mouse transplantations
Seven- to 9-week-old sublethally irradiated (3.5 Gy) female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice received a transplant of UCBMNCs or FACS-purified UCB (see “Methods”) together with at least 5 × 106 irradiated UCBMNCs by lateral tail vein injection.17 Ten weeks after transplantation, human hematopoietic cell engraftment was assessed by immunolabeling with antimouse CD45-FITC and antihuman CD45-PE. Further characterization of donor cells was performed by labeling with antibodies to human CD3, CD4, CD19, CD20, CD33, CD34, CD38, CD15, CD14, and CD59 (BD Biosciences, San Jose, CA).
Immunohistochemistry
Embryonic tissues were fixed in 4% paraformaldehyde and stained with CD34 (QBEND/10; Serotec, Dusseldorf, Germany) and CD45 (Hle-1; Becton Dickinson) as described.7 The BB9 mAb was revealed using the tyramide signal amplification system (TSA; NEN-Perkin Elmer, Wellesley, MA) according to the manufacturer's recommendations. Slides were counterstained with hematoxylin (Sigma-Aldrich, St Louis, MO), mounted in XAM neutral medium (BDH Laboratory Supplies, Poole, United Kingdom), and examined and imaged using an Optiphot 2 microscope (Nikon, Tokyo, Japan).
Hematopoietic progenitor cell clonogenic assays
To determine the effect of ACE inhibition on colony formation, pure, FACS-sorted populations of UCB CD34+CD38− or CD34+CD38+ cells were plated in methylcellulose as described previously.6 Five hundred cells were plated in triplicate in medium containing SCF (100 ng/mL; R&D Systems, Minneapolis, MN), IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) (10 ng/mL each; R&D Systems), and 4 U/mL erythropoietin (Eprex; Janssen Cilag, Saunderton, United Kingdom). ACE inhibitor lisinopril (a kind gift from Dr Robert Nordon, University of New South Wales) was serially diluted and added to the methylcellulose at the time of plating over a final concentration range of 0.01 to 100 μg/mL. Cells were incubated at 37°C and scored after 12 days for the formation of granulocyte-macrophage colony-forming cell (GM-CFC), macrophage (M-CFC), granulocyte (G-CFC), burst-forming unit erythroid (BFU-E), and colony-forming unit mixed (CFU-Mix) colonies. Additional assays were performed in the presence of angiotensin II (Ang II; Bachem, King of Prussia, PA) at the doses indicated in Figure 7. These assays were performed in the absence of fetal bovine serum (FCS), which was replaced by a combination of 20 μg/mL low-density lipoprotein (catalog no. L-2139; Sigma-Aldrich), 5 × 10−5 M beta mercaptoethanol (BDH, Melbourne, Australia), 4 mM l-glutamine, 10 μg/mL recombinant human insulin (Novo Nordisk, Bagsvaerd, Denmark), 200 μg/mL iron-saturated human transferrin (catalog no. T2158; Sigma-Aldrich), and 1% deionized bovine serum albumin (BSA; Sigma-Aldrich).
Creation of stably expressing murine fibroblasts
To identify of the molecule bound by BB9, we used an approach initially developed by Herzenberg and colleagues (Hsu et al18 ). Briefly, mouse fibroblasts (L cells) were cotransfected with high-molecular-weight DNA derived from the BB9-expressing human leukemia cell line UT719 and pSV2neo. After selection in 1 mg/mL G418 (Geneticin; Gibco/Invitrogen, Frederick, MD), cells expressing the BB9 antigen were isolated by sequential rounds of immunoselection using antimouse Dynabeads (Dynal) and finally by FACS to select a clone exhibiting a high-level binding of BB9 for immunoaffinity purification of the corresponding cell-surface glycoprotein. Stably human somatic ACE-expressing L cells were created by cotransfection at a molar ratio of 10:1 with full-length human ACE cDNA (a generous gift from Pierre Corvol, Inserm U36, Paris, France) and pEGFP (Clontech, Palo Alto, CA) using Metafectene transfection reagent (Biontex Laboratories, Munich, Germany). Transfected cells were selected in 1 mg/mL G418 and maintained in 400 μg/mL. The highest ACE-expressing transfectants were isolated by FACS based on binding of the antihuman ACE mAb 5F120 (Chemicon, Temecula, CA) for subsequent flow cytometric analysis and immunoprecipitation with BB9.
Immunopurification, SDS-PAGE, and Western blotting
L cells stably expressing BB9 were lysed in 1% nonidet P40 (NP40) in buffer comprising 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA (pH 8.0) supplemented with “Complete” protease inhibitor cocktail (Roche Diagnostics, Boehringer Mannheim, Germany). Lysates were cleared by centrifugation at 1500g for 10 minutes at 4°C and precleared overnight by incubation with sheep anti–rat IgG magnetic beads (Dynal) on a rotary mixer. Immunoprecipitation was done by incubation of the cleared lysate with rat anti–mouse IgG1Fc magnetic beads (Dynal) prearmed with saturating amounts of BB9 or isotype control. Immunoprecipitation and sample preparation for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) were conducted as described previously6 but using reducing sample buffer. For Western blotting, primary anti-ACE Ab (N-20; Santa Cruz Biotechnology, Santa Cruz, CA) was used at 0.8 μg/mL.
Protein microsequencing
The 160-kDa band corresponding to the BB9 protein was excised from the polyacrylamide gel and digested with trypsin, and the peptides were extracted into trifluoroacetic acid and acetonitrile was then separated on a HP1100 LH microbore high-performance liquid chromatography (HPLC) apparatus (Agilent Instruments, Palo Alto, CA) with a Poly LC C8 1 × 100-mm column (Millipore, Billerica, MA) at 50°C. Microsequencing of the peptides was performed on the ABI Procise peptide sequencer using the manufacturer's recommended protocol (Applied Biosystems, Foster City, CA).
Results
BB9 identifies the somatic form of angiotensin-converting enzyme
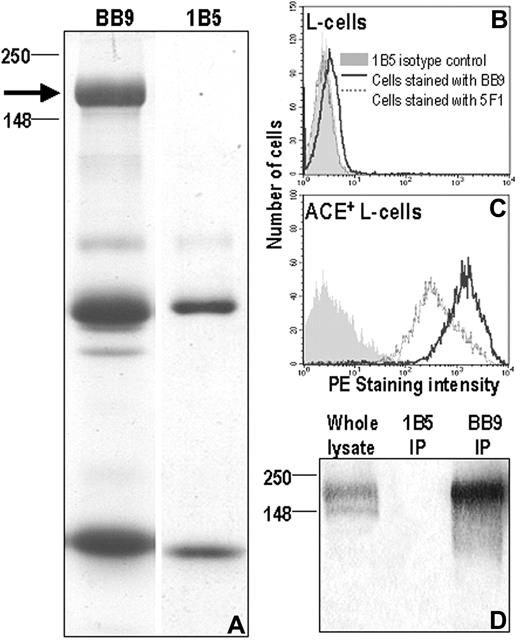
We previously demonstrated that BB9 immunoprecipitated a 160-kDa cell-surface glycoprotein from the human leukemia cell line UT7.6 Attempts to immunopurify sufficient quantities of the BB9 molecule for peptide sequencing from this cell line were unsuccessful due to the modulation of BB9 expression during bulk culture of UT7 cells. To immunopurify sufficient glycoprotein for peptide sequencing, we transfected mouse L cells with high-molecular-weight human DNA and selected cells that bound BB9 by rounds of immunomagnetic purification and FACS. Immunoprecipitation with BB9 from this line resolved a 160-kDa protein (Figure 1A) as previously reported.6 The Coomassie blue–stained band was subjected to in-gel proteolysis and the resulting peptides were resolved by HPLC and analyzed by mass spectrometry. This yielded 2 peptides, LFQELQPLYL and EADDFFTS, which demonstrated identity with peptide sequences found within the somatic form of human ACE. The identity of BB9 as ACE was confirmed by staining L cells stably expressing the full-length form of human ACE with both BB9 and a previously defined antibody to human ACE, 5F1.20 Both BB9 and 5F1 reacted specifically with ACE-transfected cells and not the parental cell line (Figure 1B,C). Immunoprecipitation and Western blotting of these ACE-expressing L cells with BB9 also yielded the expected 160-kDa glycoprotein that was specifically identified by a polyclonal ACE antibody (Figure 1D).
Specificity of mAb BB9 for human ACE. (A) Coomassie blue–stained acrylamide gel showing the 160-kDa band (left) immunopurified by mAb BB9 from BB9+ L cells and used for protein sequencing. Shown on the right is an immunoprecipitation done using nonbinding isotype control mAb 1B5. The arrow indicates the position of the BB9-specific band with respect to molecular weight markers (in kilodaltons). The smaller bands present in both lanes correspond to Ig heavy and light chain monomers. (B,C) Murine L cells stably expressing full-length human ACE cDNA and nontransfected L cells were stained with mAb BB9, anti-ACE mAb 5F1, and a nonbinding isotype control, mAb 1B5. Nontransfected cells (B) did not react with any of the antibodies while ACE-transfected L cells (C) were stained with both 5F1 and BB9. (D) Immunoprecipitates of ACE-transfected L cells with BB9 and 1B5 were Western blotted and probed with polyclonal Ab to human ACE. Note the specific reactivity with a 160-kDa protein in the BB9 immunoprecipitation and in whole lysate.
Specificity of mAb BB9 for human ACE. (A) Coomassie blue–stained acrylamide gel showing the 160-kDa band (left) immunopurified by mAb BB9 from BB9+ L cells and used for protein sequencing. Shown on the right is an immunoprecipitation done using nonbinding isotype control mAb 1B5. The arrow indicates the position of the BB9-specific band with respect to molecular weight markers (in kilodaltons). The smaller bands present in both lanes correspond to Ig heavy and light chain monomers. (B,C) Murine L cells stably expressing full-length human ACE cDNA and nontransfected L cells were stained with mAb BB9, anti-ACE mAb 5F1, and a nonbinding isotype control, mAb 1B5. Nontransfected cells (B) did not react with any of the antibodies while ACE-transfected L cells (C) were stained with both 5F1 and BB9. (D) Immunoprecipitates of ACE-transfected L cells with BB9 and 1B5 were Western blotted and probed with polyclonal Ab to human ACE. Note the specific reactivity with a 160-kDa protein in the BB9 immunoprecipitation and in whole lysate.
BB9 identifies mouse SCID-repopulating cells (SRCs)
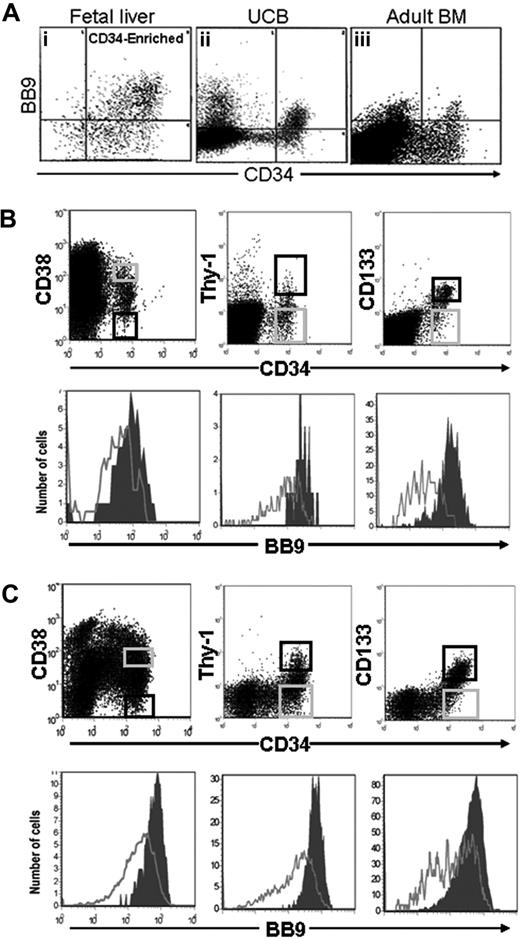
In previous studies, we showed that the BB9 recognized a subpopulation of CD34+ cells in adult human BM and cytokine-mobilized peripheral blood.6 When we analyzed the pattern of expression of BB9/ACE on primitive hematopoietic cells in human UCB (Figure 2Aii), we found that BB9 labeled 67.8% plus or minus 3.9% (n = 5) of CD34+ UCB cells, a significantly higher proportion than the 22.4% previously reported for CD34+ cells in adult BM6 (Figure 2Aiii). The highest levels of BB9 expression were observed on CD34+ cells that coexpressed CD90, CD133, and low to undetectable levels of CD38 (Figure 2B), a phenotype ascribed to HSCs in adult human hematopoietic tissues.21-24
FACS analysis of BB9 expression on subpopulations of CD34+ cells in umbilical cord blood and fetal liver. (A) The relative expression of BB9 on CD34+ cells is shown for (i) FL, (ii) UCB, and (iii) adult BM. UCB (B) and 16- to 22-week FL cells (C) were stained with BB9 and antibodies against CD34 and either CD38, Thy-1, or CD133 (top panels). The most primitive populations, CD34+CD38−, Thy-1+, CD133+ (black sort regions, top panels), express high levels of BB9 (filled histograms, bottom panels). BB9 expression on more mature CD34+ cells (gray sort regions, top panels) is represented by unfilled histograms (bottom panels).
FACS analysis of BB9 expression on subpopulations of CD34+ cells in umbilical cord blood and fetal liver. (A) The relative expression of BB9 on CD34+ cells is shown for (i) FL, (ii) UCB, and (iii) adult BM. UCB (B) and 16- to 22-week FL cells (C) were stained with BB9 and antibodies against CD34 and either CD38, Thy-1, or CD133 (top panels). The most primitive populations, CD34+CD38−, Thy-1+, CD133+ (black sort regions, top panels), express high levels of BB9 (filled histograms, bottom panels). BB9 expression on more mature CD34+ cells (gray sort regions, top panels) is represented by unfilled histograms (bottom panels).
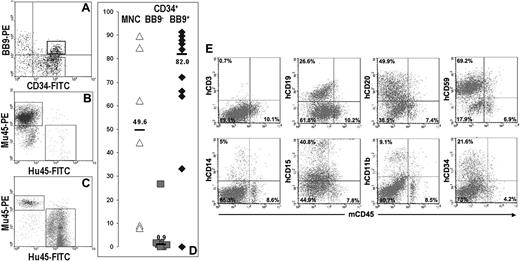
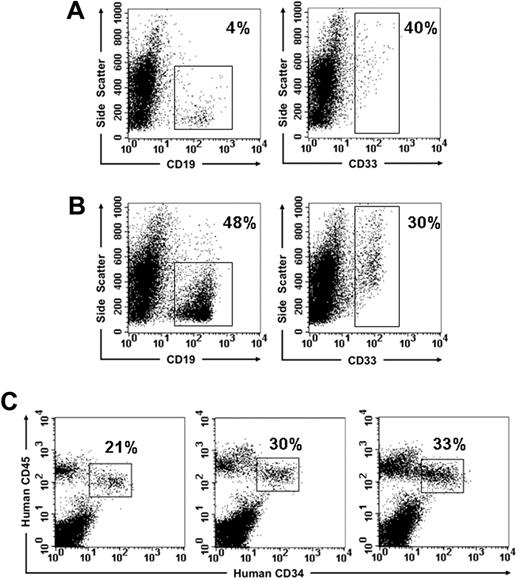
To determine whether BB9 identified cells with engrafting potential, the CD34+ fraction from UCBMNCs was separated by FACS into BB9+ and BB9− subpopulations (Figure 3A), and these 2 fractions were transplanted into multiple irradiated NOD/SCID mice at doses ranging from 7.5 × 104 to 2 × 105 cells per mouse. Unfractionated cord blood MNCs were transplanted as a control. Analysis of recipients of CD34+BB9+ cells 10 weeks after transplantation demonstrated high levels of engraftment (median: 82%; n = 11) in comparison with NOD/SCID recipients of CD34+BB9− cells, which exhibited low to undetectable levels of engraftment (median: 0.9%; n = 9; Figure 3B-D). All BB9+CD34+ recipients demonstrated multilineage engraftment as assessed by staining with antibodies to human erythroid, myeloid, and B-lymphoid markers (Figure 3E).
Human hematopoietic engraftment potential in NOD/SCID mice is restricted to CD34+ UCB cells expressing BB9. (A) Sort regions used for isolation of CD34+BB9+ ( ) and CD34+BB9− (◆) cells. (B and C) Representative examples displaying flow cytometric analysis of bone marrow from NOD/SCID recipients of CD34+BB9− and CD34+BB9+ UCB, respectively. Human (Hu45) and murine (Mu45) hematopoietic cells are identified by species-specific anti-CD45 immunolabeling. (D) Proportion of human CD45+ cells in individual animals that received a transplant of 2 × 106 unfractionated mononuclear cells (MNCs), and between 1 to 2 × 105 CD34+BB9− or CD34+BB9+ cells. — represents the median percentage of human CD45+ cells of each group. (E) Representative example of multilineage human hematopoietic engraftment in an animal that received a transplant of 1× 105 CD34+BB9+ cells. Both myeloid (CD11b, CD14, CD15) and B-lymphoid (CD19, CD20) progeny are evident.
) and CD34+BB9− (◆) cells. (B and C) Representative examples displaying flow cytometric analysis of bone marrow from NOD/SCID recipients of CD34+BB9− and CD34+BB9+ UCB, respectively. Human (Hu45) and murine (Mu45) hematopoietic cells are identified by species-specific anti-CD45 immunolabeling. (D) Proportion of human CD45+ cells in individual animals that received a transplant of 2 × 106 unfractionated mononuclear cells (MNCs), and between 1 to 2 × 105 CD34+BB9− or CD34+BB9+ cells. — represents the median percentage of human CD45+ cells of each group. (E) Representative example of multilineage human hematopoietic engraftment in an animal that received a transplant of 1× 105 CD34+BB9+ cells. Both myeloid (CD11b, CD14, CD15) and B-lymphoid (CD19, CD20) progeny are evident.
Human hematopoietic engraftment potential in NOD/SCID mice is restricted to CD34+ UCB cells expressing BB9. (A) Sort regions used for isolation of CD34+BB9+ ( ) and CD34+BB9− (◆) cells. (B and C) Representative examples displaying flow cytometric analysis of bone marrow from NOD/SCID recipients of CD34+BB9− and CD34+BB9+ UCB, respectively. Human (Hu45) and murine (Mu45) hematopoietic cells are identified by species-specific anti-CD45 immunolabeling. (D) Proportion of human CD45+ cells in individual animals that received a transplant of 2 × 106 unfractionated mononuclear cells (MNCs), and between 1 to 2 × 105 CD34+BB9− or CD34+BB9+ cells. — represents the median percentage of human CD45+ cells of each group. (E) Representative example of multilineage human hematopoietic engraftment in an animal that received a transplant of 1× 105 CD34+BB9+ cells. Both myeloid (CD11b, CD14, CD15) and B-lymphoid (CD19, CD20) progeny are evident.
) and CD34+BB9− (◆) cells. (B and C) Representative examples displaying flow cytometric analysis of bone marrow from NOD/SCID recipients of CD34+BB9− and CD34+BB9+ UCB, respectively. Human (Hu45) and murine (Mu45) hematopoietic cells are identified by species-specific anti-CD45 immunolabeling. (D) Proportion of human CD45+ cells in individual animals that received a transplant of 2 × 106 unfractionated mononuclear cells (MNCs), and between 1 to 2 × 105 CD34+BB9− or CD34+BB9+ cells. — represents the median percentage of human CD45+ cells of each group. (E) Representative example of multilineage human hematopoietic engraftment in an animal that received a transplant of 1× 105 CD34+BB9+ cells. Both myeloid (CD11b, CD14, CD15) and B-lymphoid (CD19, CD20) progeny are evident.
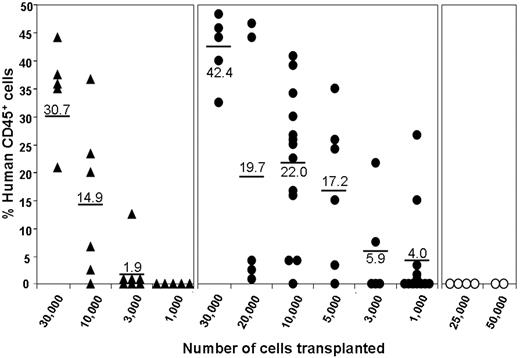
To provide a more quantitative comparison of the engrafting potential of these subpopulations, limit dilution transplantations were performed using the NOD/SCID model over a dose range of 103 to 3 × 104 cells for CD34+BB9+ cells and the unfractionated CD34+ population at doses of 2.5 × 104 and 5.0 × 104 for the CD34+BB9− subpopulation. Engraftment was measured by means of flow cytometric analysis at 10 weeks after transplantation and was defined as a level of human CD45 expression in the bone marrow of recipient mice of 1% or less. As shown in Table 1, mice that received a transplant of either dose of CD34+BB9− cells failed to engraft in agreement with the preceding transplantation studies. As anticipated, NOD/SCID mice receiving either the total CD34+ or the CD34+BB9+ subpopulation exhibited readily detectable engraftment (Table 1; Figure 4). Of the mice that engrafted following administration of CD34+BB9+ cells or the total CD34+ population, 25 and 10, respectively, were analyzed for multilineage engraftment. Irrespective of the level of CD45 cell engraftment (Figure 4), all 35 mice analyzed exhibited both myeloid (CD33+; mean: 20.2% of hCD45+ cells; range: 4%-47%) and B-lymphoid (CD19+; mean: 49.4% of hCD45+ cells; range: 16%-85%) engraftment together with a significant proportion of CD34-expressing cells (mean: 17.8%; range: 5%-55% of hCD45+ cells). Representative examples of multilineage engraftment are shown in Figure 5. Limit dilution analysis of these engraftment data demonstrates an incidence of SCID-repopulating cells (SRCs) in the CD34+BB9+ subpopulation of 1 in 2984 cells, which is significantly higher (P = .009) than that calculated for the total CD34+ population of 1 in 8376 cells. Collectively, these results identify BB9/ACE as a previously unrecognized marker of mouse SCID-repopulating cells (SRCs).22
Comparison of the in vivo reconstitution capacity of UCB-derived CD34+, CD34+BB9+, and CD34+BB9− subpopulations in the NOD/SCID mouse model
| No. of injected cells . | Proportion of engrafted mice* . |
|---|---|
| CD34+ | |
| 1 × 103 | 0/5 |
| 3 × 103 | 1/7 |
| 10 × 103 | 5/6 |
| 30 × 103 | 5/5 |
| CD34+BB9+ | |
| 1 × 103 | 5/12 |
| 3 × 103 | 2/5 |
| 5 × 103 | 5/6 |
| 10 × 103 | 12/13 |
| 20 × 103 | 5/5 |
| 30 × 103 | 5/5 |
| CD34+BB9− | |
| 25 × 103 | 0/4 |
| 50 × 103 | 0/2 |
| No. of injected cells . | Proportion of engrafted mice* . |
|---|---|
| CD34+ | |
| 1 × 103 | 0/5 |
| 3 × 103 | 1/7 |
| 10 × 103 | 5/6 |
| 30 × 103 | 5/5 |
| CD34+BB9+ | |
| 1 × 103 | 5/12 |
| 3 × 103 | 2/5 |
| 5 × 103 | 5/6 |
| 10 × 103 | 12/13 |
| 20 × 103 | 5/5 |
| 30 × 103 | 5/5 |
| CD34+BB9− | |
| 25 × 103 | 0/4 |
| 50 × 103 | 0/2 |
Engraftment is based on flow cytometric analysis of bone marrow cells from recipient mice harvested at 10 weeks after transplantation and is defined as more than 1% human CD45+ cells.
NOD/SCID-repopulating cells (SRCs) are enriched within the CD34+BB9+ subpopulation. Groups of mice received a transplant of CD34+ (▲), CD34+BB9+ (●), or CD34+BB9− cells (○) at the doses shown. Shown are the levels of human engraftment assessed by flow cytometric analysis of the bone marrow at week 10 after engraftment using anti-hCD45. The mean percentage of hCD45 in each group is indicated.
NOD/SCID-repopulating cells (SRCs) are enriched within the CD34+BB9+ subpopulation. Groups of mice received a transplant of CD34+ (▲), CD34+BB9+ (●), or CD34+BB9− cells (○) at the doses shown. Shown are the levels of human engraftment assessed by flow cytometric analysis of the bone marrow at week 10 after engraftment using anti-hCD45. The mean percentage of hCD45 in each group is indicated.
Multilineage human hematopoietic engraftment in NOD/SCID mice that received a transplant of limited numbers of CD34+BB9+ cells. Representative examples from the experiment shown in Figure 4 showing myeloid (CD33) and B-lymphoid (CD19) engraftment in individual NOD/SCID recipients of 104 (A) and 2 × 104 (B) CD34+BB9+ cells. The percentages indicate the proportion of hCD45+ cells expressing the particular lineage marker. (C) Examples of 3 mice that received a transplant of 103, 5 × 103, and 2 × 104 CD34+BB9+ cells, demonstrating by means of 2-color analysis the significant proportion of hCD45+ cells within the bone marrow represented by CD34-expressing cells.
Multilineage human hematopoietic engraftment in NOD/SCID mice that received a transplant of limited numbers of CD34+BB9+ cells. Representative examples from the experiment shown in Figure 4 showing myeloid (CD33) and B-lymphoid (CD19) engraftment in individual NOD/SCID recipients of 104 (A) and 2 × 104 (B) CD34+BB9+ cells. The percentages indicate the proportion of hCD45+ cells expressing the particular lineage marker. (C) Examples of 3 mice that received a transplant of 103, 5 × 103, and 2 × 104 CD34+BB9+ cells, demonstrating by means of 2-color analysis the significant proportion of hCD45+ cells within the bone marrow represented by CD34-expressing cells.
BB9 is expressed during the development of the human hematopoietic system
These findings prompted us to examine the temporal pattern of BB9 expression at earlier stages in the ontogeny of the human hematopoietic system. In human fetal liver, 73% ± 6.1% (n = 2) of CD34+ cells coexpressed high levels of BB9 (Figure 2Ai). As with UCB, fetal liver CD34+ cells exhibiting surrogate phenotypic characteristics of HSCs (low/undetectable expression of CD38, high CD90 and CD133) expressed the highest levels of BB9 (Figure 2C).
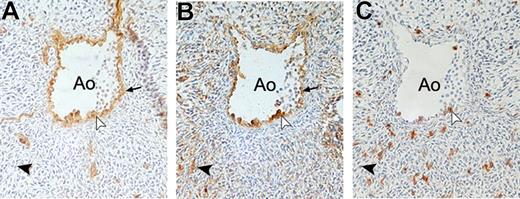
To further investigate the expression of BB9 during hematopoietic development, immunohistochemical staining was performed on 5-week-old human embryos. At 34 days of ontogeny, hematopoietic cells were typically clustered on the intravascular aspect of ventral aortic endothelium, as previously described.7 All cells within these clusters and also subjacent and neighboring endothelial cells were BB9+ and CD34+ and a proportion also coexpressed CD45 (Figure 6A-C). In addition, BB9 also identified a few cells scattered among the subaortic mesoderm. No colocalization of CD34 or CD45 was observed in these BB9-positive cells.
Expression of BB9 in the AGM region of the developing human embryo. Cross sections through the dorsal aorta (Ao) in a 34-day human embryo immunostained with CD34 (A), BB9 (B), and CD45 (C). BB9 stains hematopoietic CD34+CD45+ cell clusters associated with the endothelium on the ventral site of the aorta (white arrowheads), as well as the CD34+ endothelial cells ( ). BB9 also recognizes some cells in the mesoderm underneath the aorta (
). BB9 also recognizes some cells in the mesoderm underneath the aorta ( ). No colocalization of CD34 or CD45 antigens is observed in these BB9-positive cells (➤). Sections were observed with a Nikon Eclipse 50i light microscope with 20× magnification (20×/0.50 objective) and figures were acquired by a Nikon Digital Sight DS-2Mv camera (Nikon, Tokyo, Japan). Images were processed using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
). No colocalization of CD34 or CD45 antigens is observed in these BB9-positive cells (➤). Sections were observed with a Nikon Eclipse 50i light microscope with 20× magnification (20×/0.50 objective) and figures were acquired by a Nikon Digital Sight DS-2Mv camera (Nikon, Tokyo, Japan). Images were processed using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Expression of BB9 in the AGM region of the developing human embryo. Cross sections through the dorsal aorta (Ao) in a 34-day human embryo immunostained with CD34 (A), BB9 (B), and CD45 (C). BB9 stains hematopoietic CD34+CD45+ cell clusters associated with the endothelium on the ventral site of the aorta (white arrowheads), as well as the CD34+ endothelial cells ( ). BB9 also recognizes some cells in the mesoderm underneath the aorta (
). BB9 also recognizes some cells in the mesoderm underneath the aorta ( ). No colocalization of CD34 or CD45 antigens is observed in these BB9-positive cells (➤). Sections were observed with a Nikon Eclipse 50i light microscope with 20× magnification (20×/0.50 objective) and figures were acquired by a Nikon Digital Sight DS-2Mv camera (Nikon, Tokyo, Japan). Images were processed using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
). No colocalization of CD34 or CD45 antigens is observed in these BB9-positive cells (➤). Sections were observed with a Nikon Eclipse 50i light microscope with 20× magnification (20×/0.50 objective) and figures were acquired by a Nikon Digital Sight DS-2Mv camera (Nikon, Tokyo, Japan). Images were processed using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Perturbation of renin-angiotensin system (RAS) signaling alters the proliferation of primitive hematopoietic progenitors
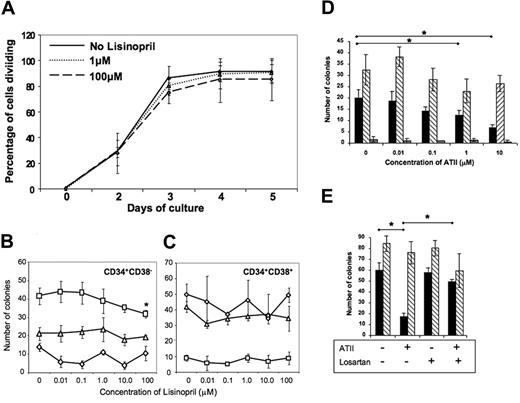
Intriguing though the expression of ACE on primitive hematopoietic cells is, this observation provides no indication of the role of ACE on these cells. To gain insight into possible function(s) of ACE on primitive hematopoietic cells, we first examined whether pharmacological inhibition of ACE activity through the addition of the specific ACE inhibitor lisinopril would alter the recruitment of primitive (CD34+CD38−) cord blood cells into division under serum-free conditions in response to a combination of early acting cytokines, SCF, thrombopoietin, and Flt 3 ligand, using a previously described single-cell recruitment assay.25 Addition of lisinopril over a 0.01- to 100-μM dose range did not alter the percentage of CD34+CD38− cells entering division over the 5-day period of the assay (Figure 7A). Although there was a trend toward lower recruitment at the 100-μM concentration of lisinopril, this did not reach statistical significance. The failure to recruit primitive cells into cycle under these assay conditions does not preclude potential affects of inhibition of ACE function on more mature clonogenic progenitors. Accordingly, we investigated the effects of lisinopril on myeloid and erythroid colony formation by CD34+CD38− cells and by their more mature CD34+CD38+ counterparts. As shown in Figure 7C, lisinopril over the same dose range as above did not alter myeloid (CFU-G, CFU-GM) or erythroid (BFU-E) colony formation by CD34+CD38+ progenitor cells. There was a similar lack of effect on CFU-GM and BFU-E colony growth by CD34+CD38− cells, but what appeared to be a dose-dependent inhibition of CFU-G colony formation, which reached statistical significance (P = .047) only at the highest dose of lisinopril (100 μM) (Figure 7B). Given the physiological role of ACE in generating angiotensin II (ATII) through the cleavage of the decapeptide angiotensin I, we finally examined the effects of ATII on colony formation by cord blood CD34+ cells. These clonogenic assays were performed in the absence of serum to avoid the presence of exogenous ACE present in FCS and the potential generation of catabolites of ATII.26 Shown in Figure 7D are data from a representative experiment (1 of 5). ATII had no effect on multipotent (CFU-Mix) colony formation nor BFU-E growth except for a minor but not statistically significant reduction in some cord blood samples. In contrast, ATII consistently inhibited myeloid (CFU-G) colony growth with highest levels of inhibition seen at the 10-μM dose. The inhibitory effect of ATII on myeloid colony formation was completely reversed in assays performed in the presence of 100 nM of the specific AGTR1 inhibitor, losartan (Figure 7E).
Pharmacological perturbation of the RAS alters the growth of hematopoietic progenitors in vitro. (A) Inhibition of ACE activity by the addition of lisinopril does not alter the recruitment into division of primitive hematopoietic cells. Single CD34+CD38− UCB cells were sorted using the ACDU of the cell sorter into 60-well Terasaki plates (Roskilde, Denmark), containing serum-free medium, SCF, TPO, and Flt3L, as described in the text, together with lisinopril at the stated doses. Two plates (up to 120 cells) were set up per lisinopril concentration. Plates were scored after 4 to 12 hours to verify the presence of single starting cells in each well and then at 24-hour intervals up to day 5 to assess the time to first division and the subsequent proliferation of recruited cells. Shown is the percentage of cells that had divided at least once plotted against days in culture. The experiment was repeated 3 times with identical results. (B,C) Lisinopril inhibits myeloid colony formation by primitive CD34+CD38− UCB cells. Clonogenic assays were established using CD34+CD38− and CD34+CD38+ UCB cells (500 cells/plate in triplicate). Colony formation was stimulated by the addition of a combination of rHu IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF, and Epo. Lisinopril was added over the dose range 0.01 to 100 μM at culture initiation. Colony formation by CD34+CD38+-derived granulocyte progenitors (CFU-Gs; □), granulocyte-macrophage-progenitors (CFU-GMs; △), or erythroid progenitors (BFU-Es; ◇) was unaffected by lisinopril. In contrast, lisinopril at 100 μM suppressed the growth of CFU-Gs derived from the more primitive CD34+CD38− population (P = .047). This figure is a representative of 3 separate experiments for both CD34+CD38− and CD34+CD38+ populations. (D) Angiotensin II inhibits myeloid (CFU-G;  ) but not erythroid (BFU-E; ▧) or CFU-Mix (
) but not erythroid (BFU-E; ▧) or CFU-Mix ( ) colony formation of UCB CD34+ cells. Clonogenic assays were established using UCB CD34+ cells (500 cells per plate in triplicate) under serum-free conditions (as described in “Hematopoietic progenitor cell clonogenic assays”) using the same combination of cytokines as described in panel C in the presence of the stated doses of angiotensin II. The asterisks indicate P < .05. Shown is one representative example of 5 replicate experiments. (E) The inhibitory effects of angiotensin II on myeloid progenitor growth in vitro is reversed by the Ang II receptor type 1 (AT1) antagonist losartan. Colony assays were established as described in panel D. Angiotensin II was used at a dose of 10 μM where indicated and losartan at 100 nM. The asterisks indicate P < .05. Shown is 1 representative experiment from a total of 3.
) colony formation of UCB CD34+ cells. Clonogenic assays were established using UCB CD34+ cells (500 cells per plate in triplicate) under serum-free conditions (as described in “Hematopoietic progenitor cell clonogenic assays”) using the same combination of cytokines as described in panel C in the presence of the stated doses of angiotensin II. The asterisks indicate P < .05. Shown is one representative example of 5 replicate experiments. (E) The inhibitory effects of angiotensin II on myeloid progenitor growth in vitro is reversed by the Ang II receptor type 1 (AT1) antagonist losartan. Colony assays were established as described in panel D. Angiotensin II was used at a dose of 10 μM where indicated and losartan at 100 nM. The asterisks indicate P < .05. Shown is 1 representative experiment from a total of 3.
Pharmacological perturbation of the RAS alters the growth of hematopoietic progenitors in vitro. (A) Inhibition of ACE activity by the addition of lisinopril does not alter the recruitment into division of primitive hematopoietic cells. Single CD34+CD38− UCB cells were sorted using the ACDU of the cell sorter into 60-well Terasaki plates (Roskilde, Denmark), containing serum-free medium, SCF, TPO, and Flt3L, as described in the text, together with lisinopril at the stated doses. Two plates (up to 120 cells) were set up per lisinopril concentration. Plates were scored after 4 to 12 hours to verify the presence of single starting cells in each well and then at 24-hour intervals up to day 5 to assess the time to first division and the subsequent proliferation of recruited cells. Shown is the percentage of cells that had divided at least once plotted against days in culture. The experiment was repeated 3 times with identical results. (B,C) Lisinopril inhibits myeloid colony formation by primitive CD34+CD38− UCB cells. Clonogenic assays were established using CD34+CD38− and CD34+CD38+ UCB cells (500 cells/plate in triplicate). Colony formation was stimulated by the addition of a combination of rHu IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF, and Epo. Lisinopril was added over the dose range 0.01 to 100 μM at culture initiation. Colony formation by CD34+CD38+-derived granulocyte progenitors (CFU-Gs; □), granulocyte-macrophage-progenitors (CFU-GMs; △), or erythroid progenitors (BFU-Es; ◇) was unaffected by lisinopril. In contrast, lisinopril at 100 μM suppressed the growth of CFU-Gs derived from the more primitive CD34+CD38− population (P = .047). This figure is a representative of 3 separate experiments for both CD34+CD38− and CD34+CD38+ populations. (D) Angiotensin II inhibits myeloid (CFU-G;  ) but not erythroid (BFU-E; ▧) or CFU-Mix (
) but not erythroid (BFU-E; ▧) or CFU-Mix ( ) colony formation of UCB CD34+ cells. Clonogenic assays were established using UCB CD34+ cells (500 cells per plate in triplicate) under serum-free conditions (as described in “Hematopoietic progenitor cell clonogenic assays”) using the same combination of cytokines as described in panel C in the presence of the stated doses of angiotensin II. The asterisks indicate P < .05. Shown is one representative example of 5 replicate experiments. (E) The inhibitory effects of angiotensin II on myeloid progenitor growth in vitro is reversed by the Ang II receptor type 1 (AT1) antagonist losartan. Colony assays were established as described in panel D. Angiotensin II was used at a dose of 10 μM where indicated and losartan at 100 nM. The asterisks indicate P < .05. Shown is 1 representative experiment from a total of 3.
) colony formation of UCB CD34+ cells. Clonogenic assays were established using UCB CD34+ cells (500 cells per plate in triplicate) under serum-free conditions (as described in “Hematopoietic progenitor cell clonogenic assays”) using the same combination of cytokines as described in panel C in the presence of the stated doses of angiotensin II. The asterisks indicate P < .05. Shown is one representative example of 5 replicate experiments. (E) The inhibitory effects of angiotensin II on myeloid progenitor growth in vitro is reversed by the Ang II receptor type 1 (AT1) antagonist losartan. Colony assays were established as described in panel D. Angiotensin II was used at a dose of 10 μM where indicated and losartan at 100 nM. The asterisks indicate P < .05. Shown is 1 representative experiment from a total of 3.
Discussion
These studies demonstrate that angiotensin-converting enzyme (ACE/CD143) as recognized by mAb BB9 is a hitherto unrecognized marker of primitive hematopoietic cells at all stages in the ontogeny of the human hematopoietic system. Numerous reports have demonstrated that HSCs in adult human tissues are enriched in a subpopulation of CD34+ cells with the cell-surface phenotype Lin−CD38−CD90+CD133+.21-24 We previously showed that BB9 reacts with cells exhibiting the candidate HSC phenotype in adult BM and cytokine-mobilized peripheral blood,6 and in the present study, we extend this observation by demonstrating expression of BB9/ACE on candidate HSCs in the human AGM, FL, and UCB. Several monoclonal antibodies to ACE have previously been developed by Danilov et al including 9B9 and 5F1,20,27 and it will be of interest to determine whether their pattern of reactivity is similar to that of BB9 as reported herein. Transplantation studies using the NOD/SCID mouse model demonstrated that CD34+BB9+ cells from UCB, but not CD34+ cells lacking expression of BB9, sustained multilineage human hematopoietic engraftment. Transplantations performed under limit dilution conditions further allowed us to quantitate the incidence of mouse SCID-repopulating cells (SRCs) in the subpopulation of CD34+ cells expressing ACE. These experiments demonstrated an approximate 2.8-fold enrichment of SRCs in the CD34+BB9+ phenotype relative to their incidence in the total CD34+ population, which, given the proportion of the CD34 population represented by BB9-expressing cells and the lack of engraftment potential of the BB9− subpopulation, allows us to calculate that SRC activity within the CD34+ cell compartment is restricted to the BB9/ACE-expressing subpopulation. These studies therefore establish BB9 as a bona fide marker of human HSCs in this most rigorous surrogate assay for HSC activity.22
Prompted by these data, we examined BB9 expression during the period of incipient hematopoiesis in the human embryo. The initial phase of hematogenesis takes place in the yolk sac during the third week of human development,28 a stage of gestation that was not available in the course of this study. Nonetheless, the yolk sac is not the sole territory of HSC emergence since, prior to the onset of liver hematopoiesis, multipotent, lymphohematopoietic cells are produced autonomously in the aorta-gonad-mesonephros (AGM) region of the embryo.7,8,29
Although hematopoietic cells emerging within the human AGM are first recognizable as clusters attached to the aortic ventral endothelium from day 27 of development, the presumptive AGM is already hematogenous in culture from at least day 19 of development,8 indicating the precocious commitment to hematopoiesis of cells present within the early intraembryonic mesoderm. The identity of these earliest forerunners of the definitive hematopoietic system has, however, remained obscure. Stringent sorting of vascular endothelial cells from the 28-day and older human AGM followed by culture in the presence of MS-5 stromal cells resulted in the production of hematopoietic cells, indicating that the intraembryonic HSCs are generated through an endothelial cell intermediate,30 as suggested by studies in the avian and murine embryos.31,32 However, before day 27, hematopoietic potential in the presumptive AGM is associated with CD34−, nonendothelial cells (M.T. et al, personal observations, April 2007), suggesting either temporally regulated induction of hematopoietic competence of endothelial cells in the embryonic aorta or the existence of an ancestral population of mesodermal precursors with hematoangiogenic potential that colonizes the ventral wall of the aorta around day 27 of development. The distribution of the BB9 antigen in the 34-day embryo reported in the present study supports the latter interpretation. These data are consistent with the hypothesis that BB9 identifies a population of CD34− mesodermal cells present in the para-aortic splanchnopleura at day 24 that subsequently migrate dorsally to colonize the ventral wall of the aorta such that by day 34, both ventral aortic endothelial cells and associated clusters of HSCs express BB9, suggesting the angiohematopoietic potential of BB9-positive splanchnopleura progenitors. Admittedly, while speculative based on the data included in the current paper, this interpretation is nevertheless supported by a strong body of additional data, which include a direct functional analysis of the proposed BB9-positive, CD34− splanchnopleura progenitors (M.T. et al, manuscript in preparation). Nonetheless, the data in the current paper demonstrate that expression of ACE/CD143 identifies primitive hematopoietic cells at all stages of the ontogeny of the human hematopoietic system, from its emergence in the AGM region to adult bone marrow.
A major finding of this report is the demonstration that BB9 identifies the somatic form of ACE. ACE (CD143) is a zinc-metalloprotease with broad substrate specificity, which, as a key component of the renin-angiotensin-system (RAS) is best known for its role in the regulation of the cardiovascular and renal systems by its production of the vasoconstrictor and mitogen angiotensin II. Somatic ACE has 2 highly homologous domains, a consequence of an ancient gene duplication, each bearing functional catalytic sites that differ in substrate specificity.11,12,14,33 The N-terminal and C-terminal domains have roughly equivalent catalytic constants for angiotensin I.33 ACE is constitutively expressed on the surface of endothelial and epithelial cells in tissues including intestinal brush borders, the proximal tubule of the kidney, and the epididymis.33,34 Although various cells of hematopoietic origin are known to exhibit ACE activity, including tissue macrophages, monocyte-derived dendritic cells, and some T cells,27,35 to our knowledge, this is the first report of ACE expression by HSCs.
The relatively restricted distribution of ACE to hierarchically more primitive cells implies an as-yet-unrecognized role for ACE in both the emergence of the hematopoietic system during ontogeny and in the steady state in adult tissues. Although the precise role of ACE in this regard remains to be determined, angiotensin II, the dominant effector peptide of the RAS, has been shown to enhance erythroid differentiation in the BM through interaction with AT1.36,37 Rodgers et al37 also showed that AT1 receptors are present on human BM CD34+CD38− and CD34+CD38+ cells and that angiotensin II increases hematopoietic progenitor cell proliferation, effects that are abolished by AT1 receptor antagonists. In the present study, angiotensin II was found to inhibit colony formation by myeloid progenitors in a dose-dependent manner, an affect blocked by the AGTR1 receptor antagonist, losartan. The inhibition of colony growth by angiotensin II seen in these studies likely reflects our choice of serum-free colony assay conditions to avoid the presence of ACE in FCS.26 Stimulatory or inhibitory effects of angiotensin II on hematopoietic colony growth have previously been attributed to the use of FCS in clonogenic assays.38 The present studies also demonstrated an approximate 25% inhibition of myeloid (CFU-G) colony growth by the ACE inhibitor lisinopril. This dose-dependent effect was observed only on progenitors derived from CD34+CD38− progenitors not on those from the CD34+CD38+ counterparts, a response in keeping with the high level of ACE expression on the former subpopulation of primitive progenitors relative to CD38+ progenitors (Figure 2).
A well-documented substrate for ACE with particular relevance to the role of this enzyme in the regulation of primitive hematopoietic cells is acetyl-N-Ser-Asp-Lys-Pro (AcSDKP). AcSDKP reversibly prevents the recruitment of normal HSCs and committed progenitors into S-phase of the cell cycle by maintaining them in the G0 phase.39,40 Present at nanomolar concentrations in the blood, AcSDKP is recognized as a physiological regulator of hematopoiesis41 whose activity in the circulation is uniquely regulated by the N-terminal catalytic center of ACE.14,42,43 The expression of ACE by HSCs thus suggests the possibility that HSCs can regulate the concentration of AcSDKP and potential of other ACE substrates within their immediate environment, thereby modulating their own response to this negative regulator. Interestingly, a recent report demonstrates that AML cells overexpress ACE/CD143,44 an observation that may at least in part explain the relative refractoriness of leukemia cells to the inhibitory effects of AcSDKP.45
Our studies demonstrating the expression of ACE by hematopoietic stem and progenitor cells come at a time of burgeoning interest in the potential role of the RAS in the physiological regulation of hematopoiesis (reviewed in Hubert et al46 ). The notion of a local RAS within the bone marrow was proposed a decade ago,47 and our observation that ACE is constitutively expressed by primitive hematopoietic cells throughout ontogeny and adulthood therefore provides support for this hypothesis by adding a critical component to this putative locally active RAS in the bone marrow. In this context, it is important to note that antibody BB9 was generated following immunization of mice with human marrow stromal cells6 and is reactive with mesenchymal stem cells (MSCs; P.J.S., unpublished data) in accord with previous reports documenting expression of ACE by rat MSCs.48 According to our data, therefore, ACE is expressed both by HSCs and a component of their microenvironmental niche. Studies by Corvol and colleagues have shown the existence of a local RAS including ACE during the development of blood islands in the chick embryo and that perturbation of the RAS modulates blood island differentiation during primitive yolk sac erythropoiesis (Savary et al49 ). While the existence of a local RAS at the analogous phase of hematopoiesis in the mammal is currently unknown, the current study demonstrating expression of ACE in the human AGM implies that a local RAS also exists within the intraembryonic sites of definitive hematopoiesis in humans. Future studies will be required to examine the expression of other components of the RAS in the embryonic para-aortic splanchnopleura and to determine their role during this critical period of incipient hematopoiesis in humans. The fact that ACE is known to target numerous non-RAS molecules by the action of its 2 catalytic sites50 and to cleave GPI-linked proteins such as Sca-1, Thy-1, and CD5950 in a manner independent of the 2 known catalytic sites51 suggests that the search for targets of the effects of ACE in hematopoiesis should not be restricted to the known RAS components.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ralph Rossi and Andrew Fryga for FACS and Kate Rutherford for mouse transplant assays. We thank Dr Dagousset for assistance with procurement of human embryos, and Prof Pierre Corvol, Inserm U36, for human ACE cDNA.
This work was supported in part by a grant from the Association pour la Recherche sur le Cancer.
Authorship
Contribution: V.J.J. designed and performed research, analyzed and interpreted data, and drafted the paper; L.S., R.D., and G.W. performed research; D.N.H. designed and performed research; I.B. drafted the paper; I.S. contributed analytical tools; B.P. interpreted data and drafted the paper; M.T. designed and performed research and interpreted data; and P.J.S. designed research, contributed methodology, interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Simmons, Director of Stem Cell Research, Brown Foundation Institute of Molecular Medicine (IMM), University of Texas Health Science Center at Houston, 1825 Pressler St, Houston, TX 77030; e-mail: paul.j.simmons@uth.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal