Abstract
The late effects of chemotherapy on immunologic parameters in AIDS-related non-Hodgkin lymphoma (NHL) have not been described. From a cohort of 105 consecutive patients treated with infusional chemotherapy and highly active antiretroviral therapy (HAART), 68 survived more than 3 months following the end of chemotherapy. Their lymphocyte subsets and plasma HIV viral loads were measured at regular intervals for 2 years and values compared with baseline. During chemotherapy, there were statistically significant falls in CD4 (helper T), CD8 (cytotoxic T), and CD19 (B) cell populations but no changes in the CD56 (natural killer [NK]) cell population. Among the 68 survivors, there were statistically significant increases in CD4, CD8, CD19, and CD56 cell populations during the first year of follow up, compared with the values at the start of chemotherapy. During the second year of follow up, there were further statistically significant rises in CD4 and CD19 cell populations, compared with the values at 12 months after chemotherapy. During 244 years of follow-up since chemotherapy in these 68 survivors, 7 second primary tumors and 8 opportunistic infections were diagnosed. Chemotherapy and concomitant HAART for AIDS-related NHL does not cause prolonged suppression of lymphocyte subsets. These data should provide reassurance regarding the long-term consequences of chemotherapy in these individuals.
Introduction
The last decade has shown dramatic improvements in the management of AIDS-related non-Hodgkin lymphoma (NHL). Studies from the post-HAART (highly active antiretroviral therapy) era report response rates and short-term survival rates approaching those in immunocompetent patients.1-8 A new prognostic index for AIDS-related NHL confirms this and demonstrates that the international prognostic index (IPI), which was established for aggressive NHL in the immunocompetent population, is also a valuable predictor of survival in AIDS-related NHL, especially when combined with CD4 cell count at the time of lymphoma diagnosis.9 Despite these encouraging results, malignancies including lymphoma are emerging as one of the major causes of death in patients with HIV who have access to HAART.10,11
Most published series describe the outcome of treatment of AIDS-related NHL with relatively short follow-up and there remain concerns over the late effects of chemotherapy in people with AIDS especially the late effects on immunologic parameters. Indeed, studies in the pre-HAART era suggested persistent severe immunosuppression following chemotherapy for AIDS-related NHL.12 These worries remain and were highlighted by the results of AIDS malignancies consortium trial 010, comparing CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab with CHOP (R-CHOP) in 149 patients with AIDS-related NHL.13 This study revealed an excess of deaths due to infection after completion of chemotherapy especially among patients with very low CD4 cell counts (< 50 cells/mL) enrolled to receive R-CHOP with maintenance rituximab whose lymphoma was in remission. We do not know, however, whether these complications were therapy related or whether they were due to prior advanced immunosuppression.
We have previously reported that treatment with concomitant HAART and chemotherapy for AIDS-related lymphoma resulted in lymphocyte subset suppression during chemotherapy and suggested early restoration of CD4 cell counts after completing chemotherapy.14 We sought to assess the late immunologic impact of chemotherapy on a cohort of patients successfully treated for AIDS-related NHL.
Methods
Patients
All patients diagnosed with HIV-related high-grade NHL at our institution between 1999 and 2006 were treated with a combination of infusional CDE (cyclophosphamide, doxorubicin, and etoposide) chemotherapy,15 and HAART (highly active antiretroviral therapy) comprising a combination of at least 3 antiretroviral agents including a nucleoside analog backbone combined with a protease inhibitor and or a nonnucleoside reverse transcriptase inhibitor. All patients had histologically confirmed aggressive high-grade NHL and full staging at diagnosis. The immunologic analysis here focuses on individuals surviving for more than 3 months after completing the course of chemotherapy, and this study was approved by the Chelsea and Westminster institutional review board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Follow-up and immune subsets
Following completion of chemotherapy, patients were seen at least once a month for the first year and thereafter at least once every 3 months. Lymphocyte subsets and plasma HIV-1 viral load were measured at the start of chemotherapy, after 1 and 3 months on chemotherapy, at the completion of chemotherapy, 1 month after completing chemotherapy, and thereafter at 3, 6, 12, 18, and 24 months after chemotherapy. Opportunistic infection prophylaxis was stopped following chemotherapy when the CD4 cell count achieved appropriate levels, while all patients continued on their HAART.
Total lymphocyte and subset analysis was performed using whole blood stained with murine antihuman monoclonal antibodies to CD4 (T helper cells), CD8 (a cytotoxic T-cell marker), CD19 (B cells), and CD16/56 (natural killer cells) (TetraOne; Beckman Coulter, High Wycombe, United Kingdom) and were evaluated on an Epics XL-MCL (Beckman Coulter) multiparametric 4-color flow cytometer. Plasma viral loads (Quantiplex HIV RNA 3.0; Chiron, Halstead, United Kingdom) were recorded with a lower limit of detection of 50 copies/mL.
Statistical methods
Median and interquartile ranges for lymphocyte subsets are described, and the variation of lymphocyte subsets in subjects over time is analyzed by paired t test statistics. We used Mann-Whitney U test to compare nonparametric variables between groups.
Results
Patient characteristics
Between January 1999 and December 2006, 105 patients with high-grade HIV-associated NHL were treated with CDE chemotherapy and HAART. The characteristics at presentation of the entire cohort and the study group of 68 survivors are shown in Table 1. As expected, the survivors had statistically significantly lower risk IPI scores whether measured as composite IPI risk groups or individual variables Eastern Cooperative Oncology Group [ECOG] performance score, stage, elevated serum lactate dehydrogenase (LDH) level, although not number of extranodal sites). In addition, CD4 cell count at NHL diagnosis was significantly higher in the survivors.
Patient characteristics at lymphoma presentation of entire cohort and comparison with study group who survived more than 3 months after chemotherapy
| . | All . | Survivors, more than 3 mo after chemotherapy . | P . |
|---|---|---|---|
| No. of subjects | 105 | 68 | — |
| Male, no./total (%) | 92/105 (88) | 61/68 (90) | χ2P =.38 |
| Median age, y (range) | 43 (22-77) | 41 (22-68) | MW U test P =.33 |
| Prior AIDS-defining illness, no./total (%) | 13/105 (12) | 9/68 (13) | χ2P =.71 |
| Median CD4, per mm3 (range) | 162 (2-814) | 178 (8-636) | MW U test P =.042 |
| On HAART at NHL diagnosis, no./total (%) | 62/105 (59) | 40/68 (59) | χ2P =.95 |
| Undetectable plasma HIV viral load at diagnosis, no./total (%) | 28/105 (27) | 21/68 (31) | χ2P =.19 |
| Stage III/IV, no./total (%) | 87/105 (83) | 53/68 (78) | χ2P =.07 |
| More than 1 extranodal site, no./total (%) | 52/105 (49) | 29/68 (43) | χ2P =.056 |
| EGOC score higher than 1, no./total (%) | 48/105 (46) | 22/68 (32) | χ2P =.002 |
| LDH level raised, no./total (%) | 72/105 (69) | 41/68 (60) | χ2P =.013 |
| IPI, no. (%) | — | — | χ2P =.002 |
| Low | 22 (21) | 19 (28) | — |
| Low intermediate | 26 (25) | 20 (29) | — |
| High intermediate | 30 (29) | 19 (28) | — |
| High | 27 (26) | 10 (15) | — |
| . | All . | Survivors, more than 3 mo after chemotherapy . | P . |
|---|---|---|---|
| No. of subjects | 105 | 68 | — |
| Male, no./total (%) | 92/105 (88) | 61/68 (90) | χ2P =.38 |
| Median age, y (range) | 43 (22-77) | 41 (22-68) | MW U test P =.33 |
| Prior AIDS-defining illness, no./total (%) | 13/105 (12) | 9/68 (13) | χ2P =.71 |
| Median CD4, per mm3 (range) | 162 (2-814) | 178 (8-636) | MW U test P =.042 |
| On HAART at NHL diagnosis, no./total (%) | 62/105 (59) | 40/68 (59) | χ2P =.95 |
| Undetectable plasma HIV viral load at diagnosis, no./total (%) | 28/105 (27) | 21/68 (31) | χ2P =.19 |
| Stage III/IV, no./total (%) | 87/105 (83) | 53/68 (78) | χ2P =.07 |
| More than 1 extranodal site, no./total (%) | 52/105 (49) | 29/68 (43) | χ2P =.056 |
| EGOC score higher than 1, no./total (%) | 48/105 (46) | 22/68 (32) | χ2P =.002 |
| LDH level raised, no./total (%) | 72/105 (69) | 41/68 (60) | χ2P =.013 |
| IPI, no. (%) | — | — | χ2P =.002 |
| Low | 22 (21) | 19 (28) | — |
| Low intermediate | 26 (25) | 20 (29) | — |
| High intermediate | 30 (29) | 19 (28) | — |
| High | 27 (26) | 10 (15) | — |
MW U indicates Mann-Whitney U test; IPI, international prognostic index; and —, not applicable.
Survival data
Overall, 42 patients have died and 63 survive at a median follow up of 3.8 years, and the 5-year overall survival is 59% (95% CI: 49% to 68%). Twenty-two patients died during the planned cycles of chemotherapy, 14 from progressive lymphoma, 7 from sepsis (4 during their first 2 cycles of chemotherapy), and 1 from a cerebrovascular accident. Seventeen patients have died of recurrent lymphoma, including 8 with leptomeningeal disease recurrence. Three patients have died while in remission of lymphoma; the causes of these deaths are as follows: 1 gastric cancer second primary malignancy, 1 esophageal cancer second primary malignancy, and 1 cardiac event.
Immunologic effects during chemotherapy
During the course of chemotherapy and 1 and 3 months following completion of the chemotherapy, patients had lymphocyte subsets and HIV plasma viral loads measured. From the initial cohort of 105 patients, 68 survived to the 3-month postchemotherapy time point, thus the number of subjects at each time point diminishes and this may confound interpretation of results. During chemotherapy, there were statistically significant declines in the CD4 (helper T cell), CD8 (cytotoxic T cell), and CD19 (B cell) cell populations in the peripheral blood but no significant change in the CD56 (natural killer cell) population (Table 2). At diagnosis of lymphoma, 62 (59%) of 105 patients were on HAART and the remaining patients all started HAART with their chemotherapy. At the start of chemotherapy, only 27% patients had an undetectable HIV plasma viral load. This value rose steadily through the course of chemotherapy to 69% at the completion of chemotherapy and on to 79% 3 months after completing chemotherapy. This rise in the proportion of patients with undetectable viral loads will be mainly because 41% of the cohort were antiretroviral naive and started HAART with their chemotherapy.
Changes in HIV plasma viral load and lymphocyte subsets during and shortly after CDE chemotherapy (chemo) for entire cohort of 105 patients
| . | Median value at start of chemo (IQR) . | Median change from baseline at 1 mo on chemo (IQR) . | Median change from baseline at 3 mo on chemo (IQR) . | Median change from baseline at end of chemo (IQR) . | Median change from baseline at 1 mo after chemo (IQR) . | Median change from baseline at 3mo after chemo (IQR) . |
|---|---|---|---|---|---|---|
| Subjects, no. | 105 | 97 | 88 | 81 | 73 | 68 |
| CD4 cell count, per mL | 162 (66-269) | −15 (−75-+36); P = .16 | −29 (−95-+33) P = .006 | −41 (−129-+16); P < .001 | −30 (−770-+45); P = .18 | +14 (−59-+72); P = .59 |
| CD8 cell count, per mL | 664 (462-984) | −120 (−367-+71); P < .001 | −294 (−567-+42); P < .001 | −308 (−589-+20); P < .001 | −84 (−422-+142); P = .054 | +88 (−279-+364); P = .64 |
| CD19 cell count, per mL | 86 (47-170) | −63 (−144-−18); P < .001 | −75 (−165-−39); P < .001 | −80 (−168-−42); P < .001 | −69 (−138-−20); P < .001 | −8 (−62-+29); P = .14 |
| CD56 cell count, per mL | 59 (29-116) | −6 (−34-+15) P = .49 | −2 (−44-+22); P = .12 | −10 (−44-+28); P = .059 | +5 (−25-+27); P = .41 | +7 (−22-+29); P = .97 |
| Percentage HIV VL less than 50 copies/mL | 27 | 36 | 57 | 69 | 74 | 79 |
| . | Median value at start of chemo (IQR) . | Median change from baseline at 1 mo on chemo (IQR) . | Median change from baseline at 3 mo on chemo (IQR) . | Median change from baseline at end of chemo (IQR) . | Median change from baseline at 1 mo after chemo (IQR) . | Median change from baseline at 3mo after chemo (IQR) . |
|---|---|---|---|---|---|---|
| Subjects, no. | 105 | 97 | 88 | 81 | 73 | 68 |
| CD4 cell count, per mL | 162 (66-269) | −15 (−75-+36); P = .16 | −29 (−95-+33) P = .006 | −41 (−129-+16); P < .001 | −30 (−770-+45); P = .18 | +14 (−59-+72); P = .59 |
| CD8 cell count, per mL | 664 (462-984) | −120 (−367-+71); P < .001 | −294 (−567-+42); P < .001 | −308 (−589-+20); P < .001 | −84 (−422-+142); P = .054 | +88 (−279-+364); P = .64 |
| CD19 cell count, per mL | 86 (47-170) | −63 (−144-−18); P < .001 | −75 (−165-−39); P < .001 | −80 (−168-−42); P < .001 | −69 (−138-−20); P < .001 | −8 (−62-+29); P = .14 |
| CD56 cell count, per mL | 59 (29-116) | −6 (−34-+15) P = .49 | −2 (−44-+22); P = .12 | −10 (−44-+28); P = .059 | +5 (−25-+27); P = .41 | +7 (−22-+29); P = .97 |
| Percentage HIV VL less than 50 copies/mL | 27 | 36 | 57 | 69 | 74 | 79 |
IQR indicates interquartile range.
All P values were calculated by paired t tests.
Late immunologic effects following chemotherapy
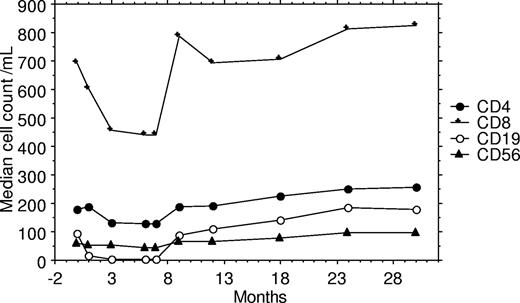
Lymphocyte subset and plasma HIV viral load data are available for 68 patients 3 months after completing chemotherapy, and further measurements of these factors has been undertaken at 6 (n = 64), 12 (n = 54), 18 (n = 47), and 24 (n = 43) months after completing chemotherapy. For the analysis of late immunologic toxicity among these survivors, only these 68 patients are included to eradicate any bias. During the first year of follow-up from the end of chemotherapy, there were statistically significant rises in CD4, CD8, CD19, and CD56 cell counts and the undetectable rate rose from 69% to 81%. The rises in cell counts were most marked during the first 3 months of follow-up, and the median rise in cell counts over the first year was 97 cells/mL for CD4, 318 cells/mL for CD8, 137 cells/mL for CD19, and 32 cells/mL for CD56. During the second year of follow-up, further statistically significant rises were documented for CD4 and CD19 cell counts but not for CD8 or CD56 cell counts (Table 3; Figure 1). Data are available for 43 patients at 24 months after chemotherapy, and for these patients the CD4 cell count rose from 165/mL (IQR: 62-252) at the start of chemotherapy to 270/mL (IQR: 174-445) 24 months after completing chemotherapy.
Changes in HIV plasma viral load and lymphocyte subsets after chemotherapy for study group of 68 patients who survived more than 3 months after completing chemotherapy (chemo)
| . | First year following chemo . | Second year following chemo . | ||||||
|---|---|---|---|---|---|---|---|---|
| Median value at start of chemo (IQR) . | Median value at end of chemo (IQR) . | Median change from end of chemo at 1 mo after chemo (IQR) . | Median change from end of chemo at 3 mo after chemo (IQR) . | Median change from end of chemo at 6 mo after chemo (IQR) . | Median change from end of chemo at 12 mo after chemo (IQR) . | Median change from 12 mo to 18 mo after chemo (IQR) . | Median change from 12 mo to 24 mo after chemo (IQR) . | |
| Subjects, no. | 68 | 68 | 68 | 68 | 64 | 54 | 47 | 43 |
| CD4 cell count, per mL | 178 (66 to 269) | 127 (66 to 269) | +15.5 (−8.5-+97); P = .001 | +47 (+13-+122); P < .001 | +69 (+14-+134); P < .001 | +97 (+54-+193); P < .001 | +33 (−27-+81); P = .030 | +48 (+3-+128); P < .001 |
| CD8 cell count, per mL | 693 (462 to 984) | 440 (66 to 269) | +130 (−30-+404); P < .001 | +279 (+77-+565); P < .001 | +243 (+77-+446); P < .001 | +318 (+110-+562); P < .001 | −21 (−188-+221); P = .85 | +14 (−160-+244); P = .32 |
| CD19 cell count, per mL | 95 (47 to 170) | 3 (66 to 269) | +3.5 (0-+33); P < .001 | +80 (+38-+126); P < .001 | +102 (+56-+155); P < .001 | +137 (+85-+256); P < .001 | +10 (−32-+66); P = .055 | +34 (−11-+93); P = .004 |
| CD56 cell count, per mL | 59 (29 to 116) | 43 (66 to 269) | +4 (−6-+33); P = .066 | +7 (−8-+39); P = .005 | +15 (−8-+60); P < .001 | +32 (−3-+89); P < .001 | −4 (−25-+25); P = .94 | +12 (−36-+4); P = .89 |
| . | First year following chemo . | Second year following chemo . | ||||||
|---|---|---|---|---|---|---|---|---|
| Median value at start of chemo (IQR) . | Median value at end of chemo (IQR) . | Median change from end of chemo at 1 mo after chemo (IQR) . | Median change from end of chemo at 3 mo after chemo (IQR) . | Median change from end of chemo at 6 mo after chemo (IQR) . | Median change from end of chemo at 12 mo after chemo (IQR) . | Median change from 12 mo to 18 mo after chemo (IQR) . | Median change from 12 mo to 24 mo after chemo (IQR) . | |
| Subjects, no. | 68 | 68 | 68 | 68 | 64 | 54 | 47 | 43 |
| CD4 cell count, per mL | 178 (66 to 269) | 127 (66 to 269) | +15.5 (−8.5-+97); P = .001 | +47 (+13-+122); P < .001 | +69 (+14-+134); P < .001 | +97 (+54-+193); P < .001 | +33 (−27-+81); P = .030 | +48 (+3-+128); P < .001 |
| CD8 cell count, per mL | 693 (462 to 984) | 440 (66 to 269) | +130 (−30-+404); P < .001 | +279 (+77-+565); P < .001 | +243 (+77-+446); P < .001 | +318 (+110-+562); P < .001 | −21 (−188-+221); P = .85 | +14 (−160-+244); P = .32 |
| CD19 cell count, per mL | 95 (47 to 170) | 3 (66 to 269) | +3.5 (0-+33); P < .001 | +80 (+38-+126); P < .001 | +102 (+56-+155); P < .001 | +137 (+85-+256); P < .001 | +10 (−32-+66); P = .055 | +34 (−11-+93); P = .004 |
| CD56 cell count, per mL | 59 (29 to 116) | 43 (66 to 269) | +4 (−6-+33); P = .066 | +7 (−8-+39); P = .005 | +15 (−8-+60); P < .001 | +32 (−3-+89); P < .001 | −4 (−25-+25); P = .94 | +12 (−36-+4); P = .89 |
Percentage HIV VL less than 50 copies/mL was 31% at the start of chemotherapy; 69% at the end of chemotherapy; 75% at 1 month after chemotherapy; 79% at 3 months after chemotherapy; 81% at 6 months after chemotherapy; 87% at 12 months after chemotherapy; 87% at 18 months after chemotherapy; and 90% at 24 months after chemotherapy.
Graph of median lymphocyte subset cell counts during and following chemotherapy for 68 patients who survived longer than 3 months after completing chemotherapy.
Graph of median lymphocyte subset cell counts during and following chemotherapy for 68 patients who survived longer than 3 months after completing chemotherapy.
Delayed toxicity following chemotherapy
Sixty-eight patients were monitored during late follow-up (more than 3 months after completing chemotherapy). The total follow-up for these patients is 244 patient years. Seven patients developed second primary tumors (2 Kaposi sarcoma [KS], 1 each anal squamous cancer, esophageal cancer, renal cell cancer, skin basal cell cancer, and skin squamous cell cancer), and a further 3 patients developed cervical intraepithelial neoplasia (CIN). During this period of follow-up, 7 patients developed 8 AIDS-defining opportunistic infections: 3 nonpulmonary tuberculosis (TB), 1 each cerebral toxoplasmosis, cryptosporidiosis, visceral leishmaniasis, pneumocystis carinii pneumonia, and esophageal candidiasis (last 2 diagnoses in the same patient).
Discussion
Dramatic and prolonged T-cell depletion following the administration of chemotherapy for AIDS-related NHL was described in the pre-HAART era.16 We describe the use of infusional CDE chemotherapy with HAART in 105 patients with AIDS-associated aggressive high-grade NHL and the early and delayed immunologic sequelae of this treatment. The early recovery of lymphocyte subsets within 3 months of the end of chemotherapy to prechemotherapy levels and the continued rise of these parameters during prolonged follow-up are reassuring, suggesting that combination chemotherapy with HAART can be safely delivered without long-term immune suppression. This pattern of lymphocyte subset recovery following chemotherapy is similar, although probably swifter than that seen in the immunocompetent population.17 Moreover, during a median follow-up of 3.8 years there were relatively few AIDS-defining opportunistic infections.
We have previously described the depression of CD4 and CD19 cells during chemotherapy and HAART treatment in both lymphoma (including 10 patients who are also included in this study)14 and Kaposi sarcoma.18 This has also been documented during chemotherapy in other studies, however the findings are often complicated by the initiation of HAART with the chemotherapy in antiretroviral-naive patients, while patients already on HAART continue with it. Moreover, none of the reports compare the pre chemotherapy and postchemotherapy lymphocyte subsets and HIV viral loads in the survivors, and so all these analyses are biased by patients who fail to complete therapy. Five studies have reported changes in CD4 cell counts during chemotherapy, including 4 where patients received concomitant HAART. In 3 papers, the fall in CD4 cell count from the start to end of chemotherapy is described in 36,1 10,2 and 203 patients with declines in median or mean CD4 cell counts recorded of −47 cells/mL, −106 cells/mL, and −61 cells/mL, respectively. One paper describes a median rise in CD4 cell count from the start of chemotherapy to its maximal value of +216 cells/mL, although it is not clear when the maximum CD4 cell count was recorded in relationship to the chemotherapy.4 The fifth paper describes therapy with the DA-EPOCH (dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisolone) chemotherapy regimen used at the NCI that requires the interruption of HAART for the entire duration of chemotherapy. During DA-EPOCH chemotherapy, the CD4 count fell by a median of 189 cells/mm3 by the end of chemotherapy.19
With regard to HIV viral load, the first 3 trials found a significant decrease during chemotherapy of −1.61 log10 copies/mL1 and −2 log10 copies/mL and −2.8 log10 copies/mL3 during chemotherapy with HAART. The study describing the maximal responses at uncertain timing reports a decline in median HIV viral load from 29 000 copies/mL at the start of chemotherapy to less than 500 copies/mL.4 In contrast, DA-EPOCH therapy without concomitant HAART was associated with a median rise in viral load during chemotherapy of +0.83 log10 copies/mL.19 A further paper describes the maintenance of virologic response during the administration of both rituximab plus CDE (R-CDE) and CHOP chemotherapy, finding 68% and 84%, respectively, of patients maintained a virologic response while receiving chemotherapy.20 In this study, HIV genotype and virtual phenotype analysis was carried out on patients who suffered virologic failure, and the authors concluded that chemotherapy does not significantly increase the occurrence of new HIV resistance patterns.20
In 3 previous reports, some information is available on recovery times of lymphocyte subsets and virologic control following completion of chemotherapy. Again, these results do not control for patients who do not survive the chemotherapy. Antinori et al1 report that the median C4 cell count 6 months after chemotherapy was + 25 cells/mL higher than at the start of chemotherapy and at 9 months was 178 cells/mL higher than at the end of chemotherapy. In our previous small study of 20 patients, the median CD4 cell count was 49 cells/mL higher 3 months after completing chemotherapy than at the start of chemotherapy.14 In contrast with the DA-EPOCH regimen, HAART is recommenced at the completion of chemotherapy and the CD4+ cells took 6 to 12 months to recover to baseline values.19 HIV viral load dropped by 2.8 log10 copies/mL at 6 months after chemotherapy in one study1 and 2.6 log10 copies/mL at 3 months in another.14 With DA-EPOCH, at 3 months after chemotherapy the viral load had fallen a median of 0.61 log10 copies/mL compared with baseline levels.19 These 3 studies reporting recovery after chemotherapy, however, do not compensate for survival and compare the values for all patients starting chemotherapy with the values following chemotherapy only of the survivors. This study compensates for bias by describing the lymphocyte subsets after completion of chemotherapy separately in the 67 patients who survived more than 3 months after the end of chemotherapy, and in this study the follow-up of 3.8 years is appreciably longer than in previous published reports.
One potential shortcoming of the analysis of delayed immunologic effects in this cohort is that inevitably only those patients who survived lymphoma can be studied. Thus the rapid immune cell recovery following chemotherapy may reflect favorable outcome and it is possible that under these circumstances the immune cells may be contributing an antilymphoma effect. These data present evidence that in those individuals who survive lymphoma, the immune subsets with the strongest mechanistic antitumor data (NK and CD8 cells) are maintained, although we have not measured specific antitumor cytotoxic T lymphocytes (CTLs).
The delayed follow-up for the 68 patients who survived at least 3 months after completing chemotherapy is 244 patient years. During this follow-up, 7 developed second primary tumors. The development of Kaposi sarcoma in 2 patients may relate to the chemotherapy-induced lymphocyte suppression, as CD4 cell nadir, CD8 cell nadir,21 and B-cell nadir,22 but not natural killer cell nadir,23 correlate with the risk of developing KS. The 5 non–AIDS-defining cancers observed in this study follow-up period have been shown to occur at increased frequency in people with HIV,11 although a direct correlation with immunologic parameters has not been established.24
This analysis confirms that chemotherapy and concomitant HAART for AIDS-related NHL do not cause prolonged suppression of lymphocyte subsets and will alleviate clinicians' fears that this treatment may compromise long-term immunologic function in people with AIDS.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors contributed to the long-term follow-up and patient care of this large cohort and analyzed and provided data; M.B., B.G., T.P., and M.N. were the clinicians in overall charge and all authors conceptualized and approved the study; T.P., J.S., and M.B. wrote the final paper, which was reviewed by all the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark Bower, FRCP FRCPath, Department of Oncology, Imperial College, The Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom; e-mail: m.bower@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal