Questions about the transplantability of mesenchymal stem cells, their ability to engraft within the bone marrow of recipients, and hence their clinical usefulness have been hotly debated for several decades. In this issue of Blood, Dominici and colleagues demonstrate robust serial osteopoietic engraftment, but highlight that osteopoietic chimerism declines to negligible levels after 6 months.
Due to the presence of hemopoietic stem cells in the bone marrow, bone marrow transplantation has been successfully used to treat hematological diseases for many decades. Based on the concept that bone marrow also contains microenvironmental or mesenchymal stem cells (MSCs) with osteopoietic potential, the clinical use of bone marrow transplantation to treat bone disorders is also very appealing. Although initial studies suggested that MSCs cannot be transplanted intravenously, more recently documented animal models and preclinical studies have demonstrated that donor MSCs engraft in the bone marrow and give rise to bone and muscle.1-5 Furthermore, a series of clinical trials performed by this group,2,4,6 involving children with severe osteogenesis imperfecta, demonstrated that transplanting unmanipulated bone marrow resulted in a marked improvement in total body mineral content, accelerated linear growth, and decreased fracture rates. Similar results were evident following transplantation of purified MSCs. In all of these studies, however, the observed accelerated growth diminished over time. In this issue of Blood, Dominici and colleagues use a murine model for conducting a comprehensive set of serial transplantations to determine whether this outcome is related to the transplantability of cells with limited osteopoietic potential, or rather due to the engraftment of cells with robust potential that are nevertheless subject to regulatory influences, which prevent sustained therapeutic levels of donor osteopoiesis. This elegant study demonstrates that normal bone marrow donor stem cells with osteopoietic potential home and engraft after transplantation, but that these cells offer only limited regenerative contribution to host osteopoiesis. Although the precise mechanism for this lack of sustained contribution remains unclear, the authors use serial transplantation to suggest that it is not due to a limited cell potential, but rather results from either intrinsic cell or extrinsic microenvironmental regulation. Together with other recent studies, this work demonstrates that MSCs are transplantable intravenously, do engraft within the bone marrow, and do contribute to osteopoiesis. However, this work highlights the lack of sustained contribution in any therapeutic use of these cells as a critical issue for the future long-term treatment of bone disorders. Further studies will be required to determine what causes the lack of durable donor-derived osteopoiesis, and will hopefully lead to treatments that will allow the widespread use of marrow transplantation to treat bone disorders.
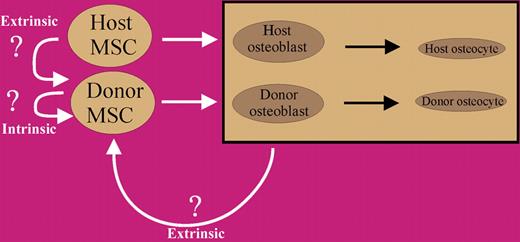
Model for donor MSC contribution to host bone formation. Initially, transplanted MSCs contribute to host bone, but after a relatively short period of time, intrinsic or extrinsic regulatory influences result in negative feedback, which causes the cells to quiesce. These cells do, however, maintain their potential to contribute to bone formation, as revealed by serial transplantation.
Model for donor MSC contribution to host bone formation. Initially, transplanted MSCs contribute to host bone, but after a relatively short period of time, intrinsic or extrinsic regulatory influences result in negative feedback, which causes the cells to quiesce. These cells do, however, maintain their potential to contribute to bone formation, as revealed by serial transplantation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal