To the editor:
Long-term follow-up of the pioneering work of Barlogie et al1 exploring the role of 2 consecutive high-dose chemotherapy cycles with autologous hematopoietic stem-cell transplantation (HSCT) in patients with myeloma has shown that a third of the patients are alive 10 years after starting protocol therapy.2
However, the question of one (1Tx) versus 2 (2Tx) high-dose chemotherapy cycles in myeloma has been a controversial one and has been discussed extensively recently.3 Two published studies have shown 2Tx to be superior to 1Tx in terms of event-free survival (EFS)4,5 and overall survival (OS),4 although the benefit seemed to be confined to patients not achieving approximately 90% cytoreduction after the first transplant. Two other studies that have been presented6,7 but not published yet suggest either comparability of 2Tx and 1Tx or an advantage of 2Tx over 1Tx. Yet another study,8 while designed as an autograft versus no autograft study, that is therapeutically more akin to a 1Tx versus 2Tx study because of the administration of nonmyeloablative high-dose melphalan to all patients, has shown superiority of the “double intensive” approach over the “single intensive” approach in terms of EFS.
In light of the findings of several large international groups, the results of the Tunisian Multiple Myeloma Study Group trial9 are puzzling. The Tunisian study showed that 1Tx followed by 6 months of thalidomide maintenance therapy was significantly superior to 2Tx without maintenance therapy in terms of OS and EFS. In addition to being somewhat counterintuitive, the findings are unique because of the extraordinarily good outcome of the 1Tx arm and the unexpectedly poor outcome of the 2Tx arm.
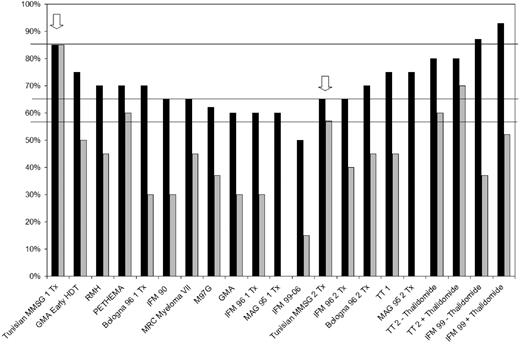
Figure 1 shows a comparison of the 3-year OS and EFS rates in the Tunisian study with those reported in some of the major published studies. The OS in the 1Tx arm of the Tunisian study is superior to the OS seen in 11 other 1Tx studies or 1Tx arms of 1Tx/2Tx studies.4,5,7,10-17 It is also higher than the OS rates reported in all 2Tx studies1,4,5,7,18 except IFM-99.19 The EFS seen in the 1Tx arm of the Tunisian study is superior to EFS ever reported in any 1Tx or 2TX study in myeloma.1-5,7,10-19
Comparison of the outcomes of the Tunisian study with the published literature. ■ represents overall survival and ▒ represents event-free survival. The arrows indicate results of the Tunisian study.
Comparison of the outcomes of the Tunisian study with the published literature. ■ represents overall survival and ▒ represents event-free survival. The arrows indicate results of the Tunisian study.
On the other hand, OS in the 2Tx arm of the Tunisian study is comparable or inferior to 6 of 11 1Tx arms/studies.5,10-14 It is comparable with that seen in one 2Tx study4 and inferior to all other 2Tx studies/arms.1,5,7,18,19 While EFS in the 2Tx arm of the Tunisian study appears to be broadly in keeping with the literature (Figure 1), the strikingly minimal difference between EFS and OS in the Tunisian suggests very short survival of relapsing patients. While this may in part be due to the lack of availability of newer agents such as bortezomib and lenalidomide,9 it is still far less than what would be expected based upon historic published data in patients who had no or limited access to thalidomide, lenalidomide, or bortezomib.1,3,11
In addition, all 12 patients undergoing allogeneic HSCT off-study (conventional-intensity conditioning with busulfan-cyclo-phosphamide; A. Abdelkefi, Centre National de Greffe de Moelle Osseuse, e-mail communication, October 2007) are alive and free of events. While this sounds extremely encouraging, the outcome of this modest number of patients is out of keeping with findings of larger studies that show much poorer outcome due to transplantation-related mortality and disease progression.20 This incidental finding should not be used to encourage widespread use of allogeneic HSCT.
While complimenting Abdelkefi et al9 on designing a good study, I would like to sound a strong note of caution before its unusual findings are used as a basis to alter clinical practice. Because of its unusual findings, not only does the study need longer follow-up, but replication of the study (or a similar concept) is essential—especially in a setting where patients have access to all appropriate salvage therapy medications.
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Jayesh Mehta, The Robert H Lurie Comprehensive Cancer Center, Northwestern University, 676 N St Clair St, Suite 850, Chicago, IL 60611; e-mail: j-mehta@northwestern.edu.